
Top news of the day from across the health care landscape.
Study shows a high burden of sexually transmitted infections among both individuals starting and persistently using PrEP.

The nasal solution is a local anesthetic indicated for the introduction of local anesthesia of the mucous membranes for diagnostic procedures and surgeries.

The proposal, which is still largely conceptual, was a part of Newsom’s proposed 2020-2021 budget.1

Sunday was National Pharmacist Day, and we asked on Facebook why you entered the pharmacy field, and why you continue to believe in the importance of the profession.
A new study published in The American Journal of Emergency Medicine found that rapid correction of elevated serum potassium levels was associated with significant reduced mortality in patients admitted to the emergency department (ED) for hyperkalemia.

Independent pharmacists have noted a growing trend in which Amazon’s Pillpack is sending unsolicited phone calls to their patients as a way of requesting transfers of prescriptions.
After injecting the flu vaccine into a tumor cell, researchers found that the injection either slowed the growth or shrunk the tumor itself.
Among eligible MSM, PrEP use is associated with lower levels of HIV anxiety, a mental health benefit that could be incorporated into initiatives that aim to increase uptake.

The diazepam nasal spray was granted 7 years of Orphan Drug Exclusivity by the FDA Office of Orphan Products Development.

The Supreme Court released their decision to review Rutledge v. Pharmaceutical Care Management Association, which could affect pharmacy benefits across the country.

Why does a patient's levothyroxine dose need to be adjusted?

Upsher-Smith Laboratories have launched haloperidol tablets, USP the generic version of the brand product, Haldol Tablets.

Will Soliman, PhD, Chairman and CEO of Accreditation Council for Medical Affairs (ACMA) discusses the exciting opportunities available to pharmacists in the pharma industry, in the second part of an interview with The Nontraditional Pharmacist.
A key demand was for the Ohio Department of Medicaid (ODM) to set a minimum reimbursement level and dispensing fees for Managed Medicaid, in order to ensure that pharmacies receive adequate reimbursement plans.

Through the Ready, Set, PrEP program, qualified patients from across the United States can access PrEP medication for the prevention of HIV, for free.

Top news of the day from across the health care landscape.

Top news of the week from Specialty Pharmacy Times.
A new study led by Johns Hopkins Medicine has shown that hundreds of deceased donor kidneys can be transplanted safely and effectively, even those discarded after being deemed unsuitable under current medical criteria.
Program allows women in sub-Saharan Africa who visited any of 16 different maternal-child health centers to receive a behavioral assessment designed to provide information about HIV risk, learn about PrEP, and obtain a prescription for PrEP, if desired.

Hemophagocytic lymphohistiocytosis (HLH) induced by lamotrigine was recently an active topic on social media, with users following a recent article that reported an Austin, Texas detective had died from the condition in 2018.

Do You Know Which State Was the First to Require Pharmacists to be Licensed?

This medication is the first precision therapy approved to treat a genomically defined population of patients with GIST, according to Blueprint Medicines.
Forty-eight states and the District of Columbia reported mumps infections in 3252 people, from January 1 to December 6, 2019, according to the CDC.

Recent studies have shown that the HIV virus may affect the brains of children living with and exposed to the virus, even with early antiretroviral therapy (ART). HIV can disrupt neurodevelopment, which affects how children learn, reason, and function.

Top news of the day from across the health care landscape.

Micafungin injection is the first antifungal drug approved in the United States specifically for pediatric patients younger than 4 years of age with invasive candidiasis.
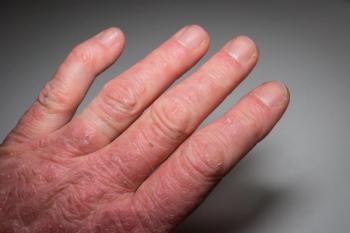
Secukinumab provided early and sustained resolution of enthesitis in patients with psoriatic arthritis based on post hoc analysis pooled data from the FUTURE trial.
Through the Automatic LifeStyle Advice platform, health service providers can integrate personal health trackers, allowing data to configure the optimal recommendation for diet, exercise, and daily health management.

Officials with the FDA have approved pembrolizumab (Keytruda, Merck) for the treatment of certain patients with Bacillus Calmette-Guerin-unresponsive, high-risk, non-muscle invasive bladder cancer.