
Sensors that can be attached to insulin pens, smart auto-injectors for patients with MS, sensors for inhalers, smart pill bottles, and bioingestible sensors are some of the evolving technologies.

Sensors that can be attached to insulin pens, smart auto-injectors for patients with MS, sensors for inhalers, smart pill bottles, and bioingestible sensors are some of the evolving technologies.

Top news of the week from Specialty Pharmacy Times.

Top news of the day from across the health care landscape.

In the third part of the series analyzing results from the Pharmacy Times Salary and Job Satisfaction Survey, we further explore pharmacists' satisfaction with their jobs and the factors that contribute to their overall happiness in a position.

Hospitalization offers unique opportunities to engage out-of-care individuals with the ultimate goal of improving their HIV outcomes and reducing health disparities.

Avapritinib was awarded FDA approval on Jan 9.

The ErbB inhibitors, gefitinib, erbstatin, and tryphostin AG825, inhibit a cell signaling process controlling the death-rate of neutrophils, immune cells, which cause damage to the lungs.

The app takes the authoritative, evidence-based 2015 World Health Organization pre-exposure prophylaxis guidelines and adapts for a mobile device.

Nexafed Decongestant Tablets are now available and in-stock from all wholesalers to relieve nasal and sinus congestion for patients.

The medication is newly indication for reducing the risk of major adverse cardiovascular events (MACE) including cardiovascular death, nonfatal heart attack, or nonfatal stroke in adults with type 2 diabetes and established cardiovascular disease (CVD).
Counties in Medicaid expansion states saw an 11% lower rate of heroin deaths and a 10% lower rate of deaths involving synthetic opioids compared with counties in non-expansion states.

Tofacitinib, currently used to treat rheumatoid arthritis and ulcerative colitis, has a correcting effect on defects that occur in inflammation.

NDMA contamination in medications has caused concern among consumers, and health care professionals after WHO recalled 1159 lots of ARBs including valsartan, losartan, and irbesartan.

Top news of the day from across the health care landscape.

Starting January 2020, aspiring certified pharmacy technicians must complete a Pharmacy Technician Certification Exam-recognized education or training program, or have equivalent work experience before taking the exam.

The statement pointed out that the spread of legalized marijuana products has increased the need for unified labeling requirements.
The emergence of a new Medicare Advantage plan, Troy Medicare, offers much needed momentum to drive pharmacists’ services.

The researchers found that the likelihood of being hospitalized for a somatic disease was just as high during the periods when the patients were on antipsychotic drugs as when they were not; however, the differences in mortality were clear.

Pharmacy students at the University of North Carolina (UNC)-Chapel Hill Eshelman School of Pharmacy are attempting to understand why clinical research takes so long to impact patient care, as well as what they can do about it in their own practices.
Belantamab mafodotin has the potential for durable responses in patients with multiple myeloma whose disease has relapsed or is resistant to standard therapies.
Top news of the day from across the health care landscape.

Upsher-Smith has launched fluvoxamine maleate tablets in 25 mg, 50 mg, and 100 mg strengths.
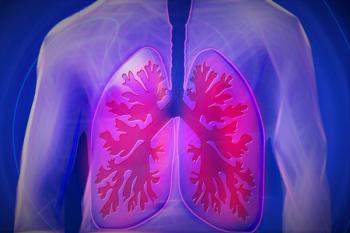
Two studies linked ozone levels and wood burning as a source of heat to an increased risk of developing chronic obstructive pulmonary disease.
The loss of immunity, also referred to as “HIV-associated immune amnesia,” could be related to why people with HIV who are on ART still have shorter lives on average than people without HIV, the study noted.
Dosing based around the frequency of sexual activity provides adequate protection for men vulnerable to HIV infection.

Lower-priced versions of Humalog Mix75/25 KwikPen and Humalog Junior KwikPen will be available in mid-April and will have 50% lower list prices compared with branded versions.
Influenza B has been causing severe illnesses in many areas of the country.
The rise in value-based reimbursement models has required oncology care providers to assume an unprecedented level of accountability.

The USP is a set of practice and quality standards for handling hazardous drugs (HDs) developed by the USP as a part of a compendium of standards related to the compounding of drugs.