Remdesivir demonstrated a statistically significant 87% reduction in risk for the composite primary endpoint of COVID-19-related hospitalization or all-cause death by day 28.

Remdesivir demonstrated a statistically significant 87% reduction in risk for the composite primary endpoint of COVID-19-related hospitalization or all-cause death by day 28.
Ten quiz questions to assess your knowledge on common symptoms and treatments for genital herpes.

It demonstrated 93.2% efficacy 14 days afterward and greater than 90% for 4 or more months.
Crizanlizumab is a humanized IgG2 kappa monoclonal antibody that binds to P-selectin and blocks interactions with its ligands.

ALS results in muscles that are reduced in size (atrophic), weak, and soft, or muscles that are stiff, tight, and spastic.

The Pharmacy Times® Pharmacy Focus podcast provides the latest industry news and information, thought-leader insights, clinical updates, patient counseling tools, and innovative solutions for the everyday practice and business of pharmacy.
DEA seizure of large-scale narcotics points to potential drug diversion in supply chain.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, provides an overview of key trials that have been conducted in the advanced cutaneous T cell lymphoma space in recent years.

Survey results from Kantar show that people rate these factors more important than an active family life and financial security in terms of well-being.

Ideas for potential new services are often plentiful, but these ideas must be carefully evaluated in order to select the right one and see it to fruition.
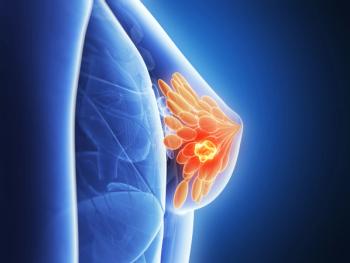
Patients with triple-negative breast cancer who received sacituzumab govitecan had a median progression-free survival of 4.6 months compared to 2.3 months with chemotherapy.

In part 2 of their discussion on COVID-19 vaccine mandates, experts Ned Milenkovich, PharmD, and Ron Lanton III, Esq., discussed how provider status could impact potential legal issues around COVID-19 vaccine mandates.
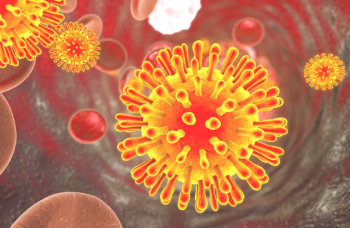
Khalili and his colleagues have completed preclinical studies that have shown that EBT-101 can effectively excise HIV proviral DNA from the genomes of different cells and tissues, including HIV-infected human cells and cells and tissues of humanized mice.
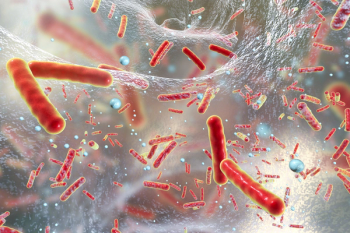
Several studies have shown that a pharmacist’s presence on a sepsis team has a statistically significant impact on patient outcomes, with pharmacists taking on several roles during patient management.

Doctors and pharmacists discuss OTC and prescription treatment options and use during webcast.
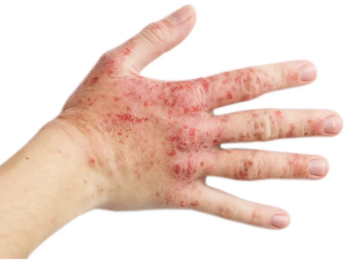
In clinical trials, the majority of patients with atopic dermatitis treated with ruxolitinib had clear or almost clear skin.
Immune globulin subcutaneous (human), 20% solution (Cuvitru) is approved for primary immunodeficiency in patients 2 years of age and older.
These patients had comparable levels of antibodies to individuals without the disease after a 2-dose course of the vaccination.

Thomas Menighan, co-founder of PursueCare and the CEO Emeritus of the American Pharmacists Association, discusses a pharmacists role in National Recovery Month.
Verquvo is a soluble guanylate cyclase stimulator indicated to reduce the risk of cardiovascular death and heart failure.

COVID-19 may change how stress for women in the pharmacy profession is viewed.

An overview of the four main types of allergies.
Macrolide resistance varied by US region but was greater than 25% in most areas and 39.5% overall.
Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, explained whether the current advanced cutaneous T cell lymphoma (CTCL) treatment strategies available are applicable for all patients with advanced CTCL and how patients are selected for treatment.
The results of 3 phase 3 trials showed that the drug offered greater proportions of individuals with the condition who met the endpoints of clinical remission and endoscopic response.

With the recent announcement of federal COVID-19 vaccine mandates, in addition to private businesses potentially requiring vaccines for employees, pharmacies and other health care facilities could have questions about legal issues surrounding these mandates.

This month's featured products include desipramine hydrochloride tablets, fingolimod capsules, and more.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, described what advanced cutaneous T cell lymphoma is in terms of classification and what current treatment approaches are available.

Trial results showed a 24% confirmed objective response rate with a median duration of response of 8.3 months among patients with cervical cancer who received tisotumab vedotin-tftv.
Relatlimab is the first LAG-3-blocking antibody to demonstrate a clinical benefit for patients with melanoma in phase 3 data.