
The president and CEO of Employers' Forum of Indiana says the pharmacists must be involved and engaged with employers to improve outcomes in health care.

The president and CEO of Employers' Forum of Indiana says the pharmacists must be involved and engaged with employers to improve outcomes in health care.
Pharmacists and the pharmacies they support are in a strong position to increase vaccination rates and address any questions related to vaccine hesitancy.

Employers can support technicians by investing in certification opportunities.

The authors of these peer -reviewed papers collectively emphasize the evolving landscape of oncology treatment.

Criticism and inquiry are coming from all directions. Will investors earn more by holding on to the past or transitioning toward the future?
The lawsuit alleges that the action was taken without the required notice and disputes the agency’s warning of “localized supply disruption,” while calling for more transparency.

Artificial intelligence (AI) is transforming drug development by automating routine tasks, enhancing clinical trials, and expediting drug discovery, ultimately leading to more personalized treatments.

Offering pharmacy professionals insights into the benefits, importance and challenges associated with switching patients to long-acting injectables to treat schizophrenia.

This Women Pharmacists Day, Jolynn Sessions, PharmD, BCOP, FHOPA, discussed the crucial role of women in pharmacy.
High-resolution scans can provide a picture of a patient’s brain that typical MRIs cannot, allowing for more detailed insights into the neurological effects of COVID-19.

Terry Keys highlights the role of language and terminology as barriers for patients receiving cancer care.
The trial met its primary endpoint of event-free survival, furthering treatment options for resected, locally advanced head and neck squamous cell carcinoma.

This approval marks the first and only anti-tissue factor pathway inhibitor approved in the US for the treatment hemophilia A or B, and the first approved hemophilia treatment to be administered via a pre-filled, auto-injector pen.
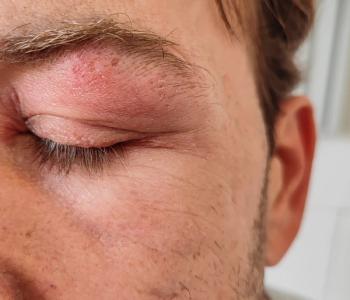
Female and older patients had a greater incidence of herpes zoster ophthalmicus and were more likely to develop ocular complications.
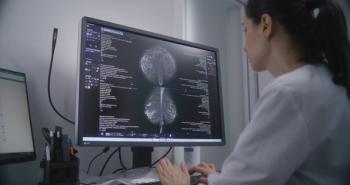
A breast-level artificial intelligence score may be able to estimate the risk of future breast cancer, allowing for patients to take preventative measures.

Amelia Arnold, PharmD, reflects on the growing recognition of pharmacists as valuable clinical providers.

Results are promising, particularly because they represent the second clinical trial of a GLP-1 receptor agonist showing potential benefits for PD motor symptoms


Pharmacists also help patients manage their adverse effects and treat patients to live healthy lives.
Sarah Hudson-Disalle, PharmD, highlights the significant impact of EHR automation on biosimilar access and costs.

Nadine Barrett, PhD, MA, MPH, discusses the role of international collaborations to advance global cancer care access.

As the first dual neurokinin-1 and 3 receptor antagonist, elinzanetant could provide benefit for women suffering from vasomotor symptoms.

The exemption allows more time for partners to adhere to enhanced distribution security requirements in the Food, Drug, & Cosmetics Act and prevents possible supply chain disruptions.

Ambulatory infusion centers can help reduce the cost of care for payers and patients and allow pharmacists to contribute to a positive treatment experience.

Naoto T. Ueno, MD, PhD, FACP, discussed the role of the Hawaiʻi Cancer Consortium to advance trial and treatment efforts for patients with cancer.

Courtney VanHouzen, PharmD shares insights about the community bispecific program at Munson Healthcare.

Oral immunotherapy is a growing area of research that involves ingesting increasing doses of the allergen to desensitize the patient’s immune system.

The FDA also approved the FoundationOne Liquid CDx assay as a companion diagnostic device to identify patients who would benefit from the treatment.
CAR T-cell therapy on an outpatient basis could lead to greater adherence and less financial and physical burdens.

The regulatory action is based on a positive preliminary analysis of the VANTAGE trial.