
Pharmacists optimize dopaminergic therapy, mitigate medication-induced psychosis, and guide antipsychotic selection to improve outcomes in Parkinson disease psychosis.

Pharmacists optimize dopaminergic therapy, mitigate medication-induced psychosis, and guide antipsychotic selection to improve outcomes in Parkinson disease psychosis.
New research highlights the efficacy and safety of HRS-1780, an investigational nonsteroidal MRA, in treating chronic kidney disease (CKD).
New findings on nodal radiation for breast cancer patients highlight the importance of lymph node involvement and tumor size in treatment decisions.

A long-term study reveals that high-fat cheese consumption may lower dementia risk, challenging traditional views on dietary fat and brain health.

Prolonged exposure to specific air pollution components significantly increases depression risk in older adults, highlighting the need for targeted regulations.
The FLEX study enhances breast cancer treatment by integrating genomic data, guiding therapy choices, and improving patient outcomes.

Research reveals that wildfire smoke exposure significantly impacts pediatric asthma control in the northeastern US, highlighting urgent climate health concerns.
Research reveals that adults who were hospitalized with RSV experience significant long-term health issues, impacting quality of life and physical function.

Pharmacists must adapt to the evolving landscape of psychedelic therapies, focusing on education, patient support, and understanding regulatory challenges.

Discover the latest FDA-approved treatments, including lumateperone for depression and elinzanetant for menopause symptoms, plus innovative health supplements.

Effective SCD pain management requires an integrated, multimodal strategy across outpatient and inpatient settings.

Pharmacists can play a pivotal role in overcoming the many challenges that exist in accessing immunomodulatory drugs in multiple myeloma.
New research explores the gut-brain connection, suggesting that improving gut health may enhance mental well-being.

Pimavanserin offers a serotonin-targeted option for Parkinson disease psychosis that improves hallucinations and delusions with minimal impact on motor symptoms.
A promising multistage malaria vaccine shows potential for broader protection against the disease, highlighting the evolving role of pharmacists in vaccination efforts.

High levels of C-reactive protein in the blood and lower heart rate variability were linked to cardiovascular events in patients with depression, anxiety, or both.

The FDA approved mitapivat, the first oral therapy for thalassemia due to anemia, offering hope for patients with transfusion-dependent and non–transfusion-dependent forms.

FDA approves mosunetuzumab's subcutaneous formulation, enhancing treatment access for relapsed follicular lymphoma.

UCSF pharmacists unveil an AI tool, Foghorn, enhancing investigational drug trials by streamlining processes and minimizing delays in drug development.

Narsoplimab gains FDA approval for treating transplant-associated thrombotic microangiopathy, showing promising survival rates in high-risk patients.

Lowering blood glucose levels significantly reduces heart disease risks in prediabetes, offering new hope for prevention and management strategies.
Sacituzumab govitecan shows promising overall survival trends in HR+/HER2− metastatic breast cancer, despite not meeting primary progression-free survival goals.

The FDA's 2025 biosimilar approvals enhanced treatment options for chronic diseases, improving accessibility and affordability for patients and health care providers.

Continuous glucose monitoring (CGM) significantly reduces the risk of high birth weight in babies born to mothers with gestational diabetes.

Discover the vital role of oncology pharmacists in cancer care, exploring their daily challenges, responsibilities, and impact on patient outcomes.
Medication errors are major public health issues, disproportionately affecting populations such as children, Ooder adults, and those with limited health literacy.

FDA approves lumateperone as a new adjunct therapy for major depressive disorder, offering hope for those with persistent symptoms despite treatment.

Six months following pneumococcal vaccination, over 70% of patients lost their protective titers, suggesting the need for more optimal vaccination strategies.
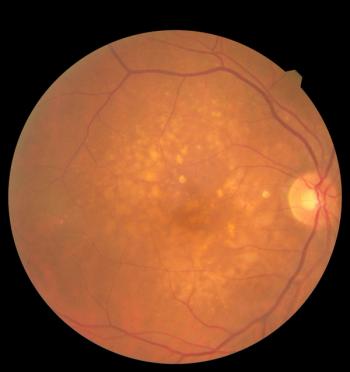
Nufymco, the FDA-approved ranibizumab biosimilar, enhances treatment options for retinal diseases, improving patient access and affordability.