Lifestyle classes, or cardiac rehabilitation, are typically offered to heart attack patients unless the patient has a particular reason why it is not suitable for them.

Lifestyle classes, or cardiac rehabilitation, are typically offered to heart attack patients unless the patient has a particular reason why it is not suitable for them.

Mathematicians at Cornell University are using game theory to model how cancer treatment may be administered more sparingly with maximized effect.

Dr. John J. Sciarra was a long time editor and contributor for Pharmacy Times during the 1980s and 1990s.

Today, we’re thanking Emily Friedman, RPh, PharmD, MBA, for her work opening testing sites and keeping her staff safe during the COVID-19 pandemic.
The findings are part of the I-SPY clinical trial and were presented at the American Association for Cancer Research virtual annual meeting.

A study to help determine the incidence rate of the coronavirus disease 2019 (COVID-19) in children and their family members has begun enrolling participants in the United States, according to the National Institutes of Health (NIH).





A rapid increase in telemedicine during the coronavirus disease 2019 pandemic could transform the way care is provided in the United States going forward.
Although lifesaving drugs became available around 1998 to treat hepatitis C, they were expensive, which limited access for some patients.

Baker and her co-authors used federal employment data to find that 10% of US workers are employed in occupations in which exposure to disease or infection happens at least weekly based on employee and employer self-reports.

The findings of a recent survey from ValuePenguin found that 55% of the 1200 respondents said their mental health is suffering because of COVID-19, more specifically in millennials (63%) and parents with younger children (64%).

CPP is a rare disease defined as the onset of puberty before age 8 years in girls and before age 9 years in boys.
Today, we’re celebrating the work of David Sullivan, PharmD, RPh, a pharmacy manager at CVS Pharmacy in Lawrence, Massachusetts.

A research team has developed wirelessly-driven “smart contact lens” technology that is able to detect diabetes and treat diabetic retinopathy just through wearing them.

What is causing a patient's outbreak of psoriasis?

The APhA’s program “Addressing the COVID-19 Crisis: An Open Forum Webinar Series for Pharmacists” addressed the new conditions, including that hand sanitizer is compounded according to the following formula consistent with World Health Organization recommendations.

The objective of the study was to examine whether positive treatment effects in cancer randomized clinical trials apply to specific demographic or insurance subgroups.
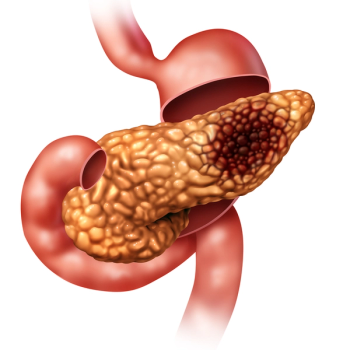
A recent study presented a risk prediction model that effectively combined genetic and clinical factors with circulating biomarkers to identify people with a higher than normal risk of pancreatic cancer.
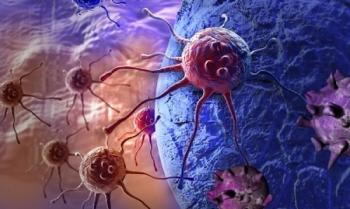
New research has discovered new elements to immunotherapy drugs that target programmed death-ligand 1 on the surface of cancer cells.
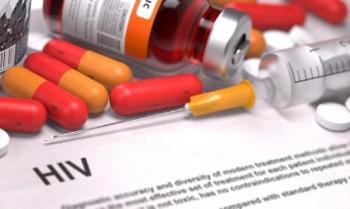
Emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets, F/TAF (Descovy, Gilead) was just as effective as emtricitabine 200 mg and tenofovir disoproxil fumarate 300 mg F/TDF (Truvada).
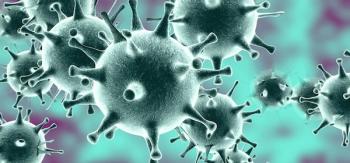
Remdesivir is not approved by the FDA. However, EUA allows for distribution and emergency use for treatment of COVID-19.
A new collaboration agreement was made between Moderna, Inc, and Lonza Ltd, to manufacture Moderna’s mRNA vaccine against the coronavirus disease 2019.

If the majority of Americans were diagnosed with COVID-19, the total cost of hospitalizations, ventilators, and other resources could cost $654 billion.

Today, we’re thanking Shady Zarnegin, PharmD, lead pharmacist at a Rite Aid in Los Angeles, California.

The relief money will be distributed on the basis of operating expenses.

There are few effective treatment options for patients with HER2-positive breast cancer who have received 2 or more previous therapies for advanced disease, according to the study authors.
The FDA has granted Bio-Rad Laboratories Emergency Use Authorization for the company’s SARS-CoV-2 Total Ab test.