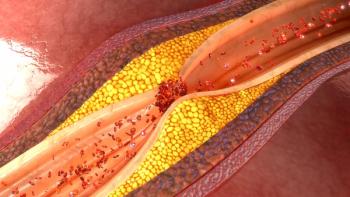
The 2025 National Lipid Association (NLA) Scientific Sessions highlighted a strategic shift toward early, aggressive cardiometabolic intervention, underscoring the vital role of pharmacists in implementing emerging therapies and optimizing cardiovascular risk reduction.