
All News


An oral dose of 0.6 mg per 5 mL of colchicine could aid individuals who have trouble with swallowing pills.
The meaningful results were reported in the DESTINY-Breast09 trial.

Although most health care professionals agree that patients do not need prescriptions for chronic diseases during end-of-life care, patients often receive them anyway.
The breakthrough designation is supported by clinical data from ongoing phase 1/2 trials.
In contrast to the study findings, the prescribing information for cefiderocol lists different common adverse effects.
Ronna Hauser, PharmD, senior vice president of policy and pharmacy affairs at NCPA, discusses concerns about the implementation of the MDPNP.

By implementing work practices alongside administrative and engineering controls, a safe and healthy work environment can be achieved.

Children who received antibiotics between birth and 2 years of age were more likely to develop food allergies, allergic rhinitis, and asthma, but not neurodevelopmental conditions.

Traditional risk factors were more contributable to cryptogenic ischemic stroke (CIS) without patent foramen ovale (PFO), whereas nontraditional ones were more critical for CIS with PFO.

Delayed pegaspargase reduces hepatotoxicity without affecting outcomes.

Philip Kuball, MD, neurology resident at NYU Langone Health, highlights the integral role of pharmacists in monitoring patient eligibility, managing dosing schedules, and ensuring safe medication interactions for lecanemab treatment in Alzheimer disease.
These results suggest that individuals who are immunocompromised with type 2 diabetes (T2D) may need multiple doses of a pneumococcal conjugate vaccine (PCV) to sustain their protection.

Lauren B. Krupp, MD, FAAN, provides comprehensive guidance on treating pediatric multiple sclerosis, emphasizing early intervention, high-efficacy therapies, and holistic family support.

Cases have been confirmed in Mexico and Canada.

Real-world data support momelotinib as an effective and well-tolerated treatment for myelofibrosis-related anemia.

The 2025 SPPCP Virtual Conference on Tuesday, May 20, 2025, will offer pharmacists and clinicians a full day of CE-accredited sessions on complex pain management, opioid use disorder, mentorship, and innovative approaches in palliative care, with opportunities for networking and professional development.

This shift brings new responsibilities and opportunities, from managing inventory changes to counseling patients on alternative therapies.

The findings suggest a potentially elevated risk of vision impairment with semaglutide (Ozempic, Wegovy; Novo Nordisk) use compared with other weight loss and diabetes medications.
FDA Approves Dupilumab, Marking First Targeted Therapy in a Decade for Chronic Spontaneous Urticaria
Multiple phase 3 clinical trials demonstrated dupilumab’s (Dupixent; Regeneron, Sanofi) effectiveness in reducing hives and itch in patients with chronic spontaneous urticaria compared with placebo.
Sequencing of antibody-drug conjugates (ADCs) in HER2-mutated breast cancer is crucial for optimizing treatment outcomes.
Authors of a review published in The Journal of Allergy and Clinical Immunology emphasized that blood eosinophil count and fractional exhaled nitric oxide cannot be relied on.

Lepodisiran, a small interfering RNA, demonstrated cholesterol-lowering effects at 16-mg, 96-mg, and 400-mg doses, with the highest dose being the most effective.
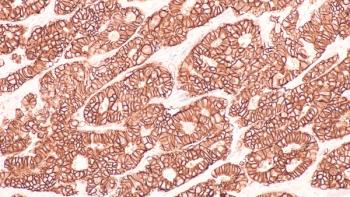
Antibody-drug conjugates have revolutionized metastatic breast cancer treatment and classification.

Hospital compounding is gaining renewed attention amid drug shortages, regulatory changes, and labor challenges, prompting pharmacies to leverage advanced technology for safer, more efficient, and compliant medication preparation.
The recommendations are not considered to be final until they are published in the Morbidity and Mortality Weekly Report.

UPMC enhances care for patients with heart failure through integrated pharmacy and cardiology team collaboration.
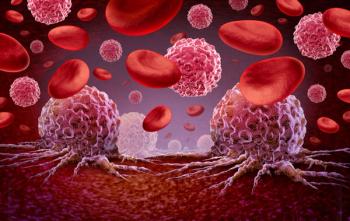
Undetectable minimal residual disease rates improved with ongoing therapy.

The implementation of hospital-based discharge pharmacy services presents a savvy strategy to improve clinical outcomes for patients while cutting excess spending.
