MONARCH 3 final OS data show that the combination of abemaciclib and a NSAI in the first-line resulted in numerically longer OS compared to an NSAI alone.

MONARCH 3 final OS data show that the combination of abemaciclib and a NSAI in the first-line resulted in numerically longer OS compared to an NSAI alone.

Individuals with HR+ breast cancer could pause endocrine therapy for up to 2 years to become pregnant, without increasing the risk of recurrence.
Patients with an estrogen receptor measure greater than 8% and those with little to no TIL expression showed no benefit with the addition of nivolumab.

The investigational combination also delayed the median time to first chemotherapy in all age groups.

Biologic rationale, biomarker precision, and efficacy results in the biomarker-negative population all helped FDA officials decide the final indication.

Evidence has shown that reversing pro-cancer effects requires significant weight loss as well as substantial metabolic reprogramming.
Patients with breast cancer who underwent chemotherapy and skipped RNI did not face an increased risk of disease recurrence or death 5 years post-surgery.
Although PFS was improved in patients with unresectable locally advanced or metastatic HER2-positive breast cancer, OS data were immature, requiring further research.
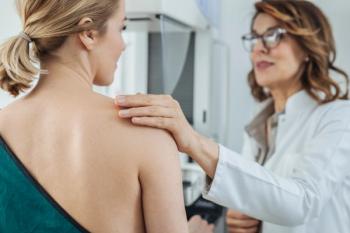
Mridula George discusses the role of personalized circulating tumor DNA monitoring in predicting the response to neoadjuvant therapy for early-stage breast cancer.
Trastuzumab deruxtecan and elacestrant both represent important new treatment options for subgroups of patients with breast cancer.

Pharmacy Times will be covering the 2023 San Antonio Breast Cancer Symposium (SABCS), happening December 5 through 9 in Texas.
Long-term data showed no difference in the clinical benefit between the treatments for HER2-negative early breast cancer with homologous recombination deficiency.

Following a phase 2 analysis of OP-1250 with palbociclib, researchers recommend an OP-1250 dose level of 120 mg/day for future trials.

Guidelines for HER2-low expressed cancer could define who and how patient recieve drugs, said expert live from San Antonio Breast Cancer Symposium 2022.

The phase 3 clinical trial shows that the 5-year OS for patients with HR+/HER2- advanced breast cancer in the abemaciclib plus fulvestrant group was 41.2% vs 29.2% for the placebo arm.

OP-1250 and palbociclib showed “enhanced suppression of tumor growth” in preclinical mouse model studies, according to expert who presented his findings in a poster at the San Antonio Breast Cancer Symposium 2022.
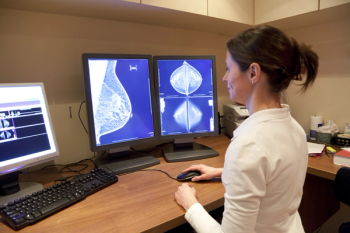
Expert sits with Pharmacy Times to share the promising results of a study that evaluated the safety of a new antibody drug conjugate with radiation therapy.
Results from the phase 3 CAPItello-291 clinical trial show that the combination doubled the median progression-free survival compared with the placebo in patients with HR-positive, HER2-negative advanced breast cancer.

BYLieve clinical trial data indicate long-term and very-long-term data disease control was observed in 25.6% and 16.5% of patients, respectively, with PFS at 24.8 and 29.4 months in patients with HR-positive, HER2-negative advanced breast cancer.
Efficacy and safety profile in combination with alpelisib is comparable to that reported in the BYLieve and SOLAR-1 clinical trials, according to a poster presentation at the San Antonio Breast Cancer Symposium.

Dawn L. Hershman, MD, MS, Columbia University Medical Center, New York, NY, presents her findings at the 2022 San Antonio Breast Cancer Symposium.
Researchers discovered the most common single gene mutation that causes treatment-resistance in first- line ER-positive or HER2-negative breast cancer treatment with a CDK4/6 inhibitor.
Clinical trial results found that second-line treatment with trastuzumab deruxtecan led to significantly longer overall survival compared with trastuzumab emtansine.
DESTINY-Breast02 clinical trial results show trastuzumab deruxtecan demonstrated a clinically meaningful and statistically significant improvement in progression-free and overall survival.
Tumor microenvironment differences in Black women may explain disparities in outcomes for those with residual tumors after chemotherapy treatment for ER–positive/HER2-negative breast cancer.

Breast cancer expert joined a panel to discuss aromatase inhibitors at the San Antonio Breast Cancer Symposium (SABCS) on December 6, 2022.
Phase 3 monarchE trial assesses distant relapse free-survival, invasive disease-free survival, and overall survival for the treatment of HR-positive/HER2-negative-, node-positive, high-risk early breast cancer.
RxPonder clinical trial results indicate that racial disparities continue to be a major health care challenge.

A new radiation therapy may reduce toxicity compared to standard therapy, paving the way for a safer and possibly, more effective, treatment of early-stage breast cancer.

Results of RIGHT Choice phase 2 clinical trial show Kisqali doubled PFS and showed fewer adverse events.