Respiratory Syncytial Virus
Latest News
Latest Videos

CME Content
More News
Differentiating between the 3 available options can help identify which is best for individual patients.

An expert on RSV discusses symptoms that warrant emergent medical care and the overall impact of RSV on older adults or immunocompromised individuals.

Cherokee Layson-Wolf, PharmD, BCACP, FAPhA, provides an overview of respiratory syncytial virus (RSV), highlighting disease transmission, incidence, symptoms, and factors that indicate higher risk of hospitalization.
Pharmacists’ awareness of respiratory syncytial virus and their role in educating about the vaccine have grown dramatically.
Carrie Koenigsfeld, PharmD, FAPhA, shares strategies that pharmacists can use to effectively tackle vaccine hesitancy, particularly concerning newer vaccines such as the RSV vaccine, through building trust with patients to encourage informed decision-making.

A medical expert examines the ideal timing for administering RSV vaccines to adults over 60 or pregnant women, emphasizing the importance of proper timing for a single-dose vaccine and outlining the necessary steps to be taken if a pregnant woman inadvertently receives an adjuvanted RSV vaccine.

The ACIP recommendations are designed to improve access to RSV vaccination, emphasizing the need to get vaccinated.

Carrie Koenigsfeld, PharmD, FAPhA, explores the potential contraindications that may preclude individuals from receiving an RSV vaccine.

Carrie Koenigsfeld, PharmD, FAPhA, highlights the main findings from the MATISSE trial, which evaluated the safety and efficacy of a non-adjuvanted RSV vaccine administered to pregnant women, presenting data that suggests the vaccine's effectiveness in preventing severe RSV in infants and discussing the observed adverse events in both mothers and infants.
Experts give important background information on respiratory syncytial virus (RSV), as well as the key clinical considerations that are associated with RSV vaccine use.

Experts give important background information on respiratory syncytial virus (RSV), as well as the key clinical considerations that are associated with RSV vaccine use.
The recent approval could further the use of mRNA technology across other indications.

An RSV medical expert provides an overview of the RENOIR trial, which investigated the efficacy and safety of an adjuvanted RSV vaccine in adults aged 60 years and older, focusing on the vaccine's effectiveness in preventing RSV-associated lower respiratory tract infections and presenting other crucial findings from the study.

Carrie Koenigsfeld, PharmD, FAPhA, reviews the pivotal findings from the AReSVi-006 trial that supported the approval of the adjuvanted RSV vaccine, including its efficacy in preventing lower respiratory tract RSV, associated adverse events, and important study limitations that may have influenced the results.

Data show that among individuals 60 years and older, the estimated rate of Guillain-Barré syndrome (GBS) after receiving an RSV vaccine was 5.0 and 1.5 per million doses of Abrysvo and Arexvy, respectively.
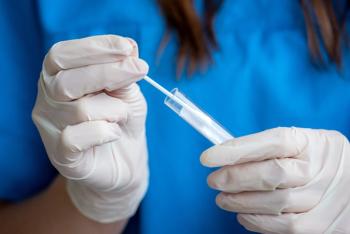
The 4-in-1 respiratory diagnostic test is either a single nasopharyngeal or anterior nasal swab that can confirm or rule out SARS-CoV-2, influenza A virus, influenza B virus, and respiratory syncytial virus with a single test.
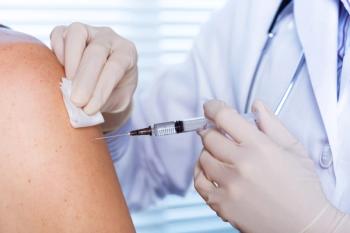
The expanded indication is for the prevention of respiratory syncytial virus-associated lower respiratory tract disease in adults aged 50 through 59 years who are at an increased risk.
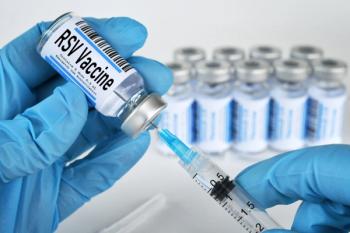
A single dose of the vaccine showed long-lasting protection against RSV-associated lower respiratory tract disease in older adults.

This is Moderna's second mRNA vaccine approval and the first mRNA vaccine approved for an indication other than COVID-19.
The study compares the safety and efficacy of mRNA-1345 compared to placebo.
Tools provide a safer testing option without using the viral proteins that cause infection.
The panelists provide guidance on how pharmacists should advise patients on recognizing RSV symptoms that require urgent medical attention.

Medical experts highlight the importance of clinical decision-making and the pivotal role pharmacies play in initiating conversations and identifying high-risk patients during consultations for new medications.

The results showed that during a 1-month hospital stay, 13% of individuals who experienced RSV-associated acute respiratory failure died.

The panelists address the challenges of administering multiple vaccines to eligible patients, while also discussing the timing and compatibility of administering certain vaccines simultaneously. They emphasize the importance of being receptive to patient concerns and providing guidance on childhood vaccinations for hesitant individuals.