New presentation offers health care providers with a more convenient, ready-to-use immunization option for the prevention of invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y, and W.

New presentation offers health care providers with a more convenient, ready-to-use immunization option for the prevention of invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y, and W.

CDC Director Rochelle Walensky, MD, MPH, announced new preferential flu vaccine recommendations for individuals aged 65 years and older.
As pharmacy moves in the direction of patient-centered care, immunization plays a key role in this shift.
Pivotal top-line data demonstrate 20vPnC, if approved likely protects against 20 serotypes in 3-dose series and may offer the broadest serotype coverage of any PCV.
Researchers have identified hydroquinine as a potential treatment against drug-resistant bacteria, which can cause infections with a 70% mortality rate.
Decision is based on interim analysis of Pfizer’s GBS6 in healthy pregnant woman aged 18 to 40 years, who were vaccinated during their second or early third trimesters.
Meropenem IV is an antibacterial medication indicated for the treatment of complicated stomach and skin structure infections.
Climatic hazards were implicated for enhancing specific aspects of pathogens, increasing capacity of pathogens to cause more severe illness, and diminishing human capacity to cope with pathogens, such as dengue, hepatitis, pneumonia, malaria, and zika.
Zerbaxa is a combination of ceftolozane, a cephalosporin antibacterial, and tazobactam, a beta-lactamase inhibitor.
A potential public health benefit of 13-valent pneumococcal conjugate vaccine is the reduction of acute chest syndrome among children with sickle-cell disease.
Moxifloxacin hydrochloride (Avelox) is indicated for treating infections in adults 18 years of age and older caused by designated susceptible bacteria.
Omadacycline (Nuzyra) is indicated for the treatment of adult patients with infections caused by susceptible microorganisms—community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections.

Piperacillin and tazobactam (Zosyn) is indicated for treatment of intra-abdominal infections, skin and skin structure infections, female pelvic infections, community-acquired pneumonia, and nosocomial pneumonia.
The most common way to detect bacteria and determine the right medication to use is with the gram stain test, which will show whether the bacteria is gram-positive or gram-negative

Pharmacists are in an excellent position to educate and counsel patients on current recommendations.
Dalbavancin (Dalvance) is indicated for acute bacterial skin and skin structure infections caused by designated susceptible strains of Gram-positive microorganisms.
The investigational PCV is designed to target serotypes responsible for 85% of all invasive cases of the disease in individuals aged 65 and older in the United States.
Oritavancin (Orbactiv) is indicated for the treatment of adult patients with acute bacterial skin and skin structure infections caused or suspected to be caused by susceptible isolates of designated Gram-positive microorganisms.
Vaxneuvance was approved for an expanded indication by the FDA on June 22, 2022, for active immunization in children 6 weeks of age and older.

Vaxneuvance is now indicated for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F in individuals 6 weeks of age and older.

New recommendations provide some harmonization to make it easier for clinicians to lower the barriers to assessing and identifying eligible patients for the pneumococcal vaccine.

Expert emphasizes that it is important to acknowledge the critical role of pharmacists in overcoming barriers to vaccinations.
Ciprodex is approved for the treatment of ear infections, acute otitis media, and acute otitis externa.
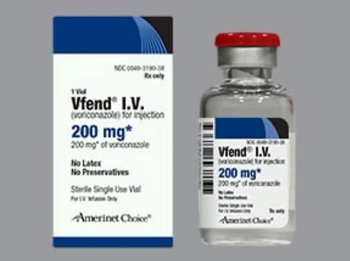
Voriconazole (Vfend) is an azole antifungal indicated for the treatment of invasive aspergillosis and Candidemia in non-neutropenics and other deep tissue Candida infections.
Any antibiotic use was associated with higher rates of IBD, and the risk went up substantially with each course.