Multiple Myeloma
Latest News
Latest Videos

CME Content
More News

Teclistamab shows promising results in frontline multiple myeloma treatment, achieving high MRD negativity rates, but safety concerns remain.
Elranatamab combined with daratumumab and lenalidomide shows promising results for treating newly diagnosed multiple myeloma, enhancing patient outcomes.

An expert discusses the benefits of anti-CD38 quadruplet regimens for elderly multiple myeloma patients.

An expert discusses CAR T therapy's potential in treating relapsed multiple myeloma, highlighting long-term remissions and access challenges.
The 2025 International Myeloma Society Annual Meeting unveils crucial updates to IMWG guidelines, enhancing response assessment and treatment strategies for multiple myeloma.
September highlights Blood Cancer Awareness Month, focusing on education, early diagnosis, and support for multiple myeloma, lymphoma, and leukemia patients.

Subcutaneous bortezomib shows fewer adverse effects in multiple myeloma treatment compared with intravenous administration, enhancing patient outcomes.

For pharmacists, understanding the evolving therapeutic landscape is essential—not only to optimize drug selection and dosing but also to counsel patients on complex regimens, manage adverse effects, and monitor for treatment-related toxicities.
A patient, who formerly had previously been treated with CAR therapy for multiple myeloma, achieved remission for both multiple myeloma and lymphoma.

New research uncovers the complex tumor microenvironment in multiple myeloma, revealing unique plasma cell ecosystems that challenge existing treatment approaches.
SWIFT-seq revolutionizes multiple myeloma diagnosis, offering a noninvasive blood test that enhances monitoring and risk assessment for patients.
CARTITUDE-1 data show one-third of patients treated with cilta-cel remain progression free at 5 years without maintenance.
Discover the latest advancements in multiple myeloma treatment, including new therapies and FDA approvals that enhance patient outcomes and survival rates.
Discontinuing lenalidomide after 3 years of MRD negativity shows low relapse rates in multiple myeloma, offering hope for treatment-free remission.

Sanofi's SAR446523 receives orphan drug designation for relapsed/refractory multiple myeloma, enhancing treatment options and patient outcomes.
Sundar Jagannath, MD, discusses how prophylactic tocilizumab reduced cytokine release syndrome incidence and severity with talquetamab in relapsed or refractory (R/R) multiple myeloma.

The advisory committee cited concerns around ocular toxicity and overall tolerability, raising serious questions about the drug's benefit-risk profile ahead of its scheduled Prescription Drug User Fee Act action date on July 23, 2025.
The National Comprehensive Cancer Network (NCCN) has released multiple myeloma guidelines version 2.2026 to include linvoseltamab as a preferred treatment for heavily pretreated patients, highlighting its intravenous formulation, dosing options, and streamlined Risk Evaluation and Mitigation Strategy program.
The data showed patients with relapsed or refractory multiple myeloma achieved a 73% overall response rate.
New data reveal isatuximab-based therapy achieves high MRD-negativity rates in newly diagnosed multiple myeloma.
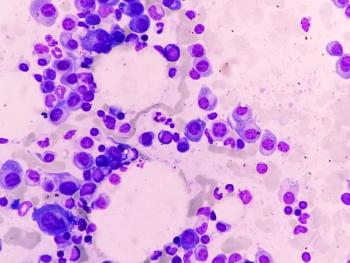
The treatment becomes the first approved BCMAxCD3 bispecific antibody with the potential to achieve biweekly or monthly dosing in patients with relapsed/refractory multiple myeloma.
CARTITUDE-1 trial reveals cilta-cel's potential in achieving long-term remission for relapsed/refractory multiple myeloma, sparking discussions on curative possibilities.
FDA removes REMS for CAR T-cell therapies, enhancing patient access while ensuring safety in cancer treatment.

The agent continues to show promising clinical benefit despite being pulled from the market in 2023.
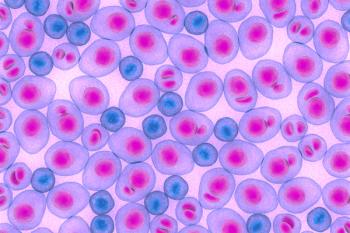
Data from a phase 1b study showed significantly reduced toxicity compared with conventional oral dosing.