Multiple Myeloma
Latest News

Latest Videos

CME Content
More News
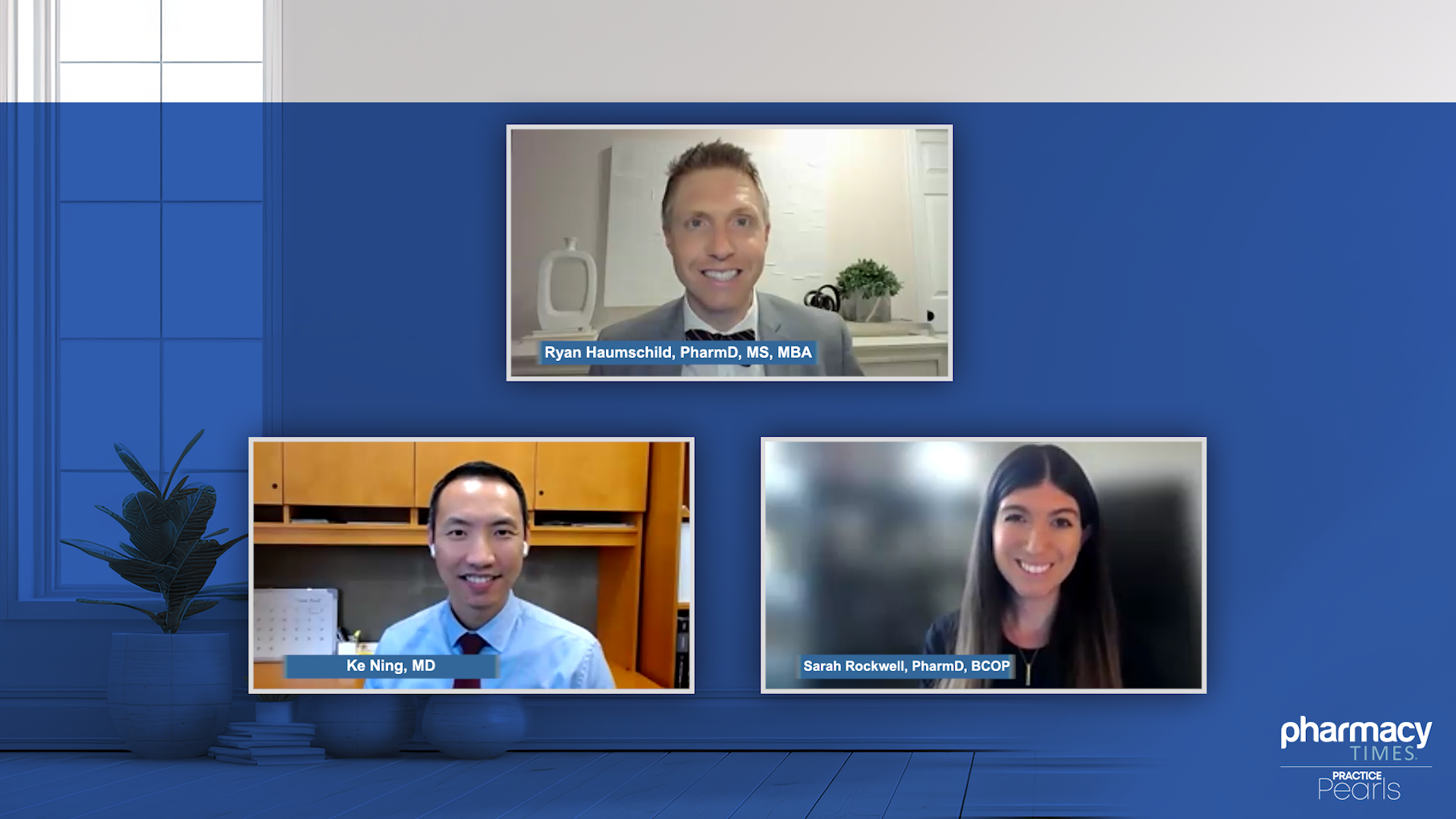
The expert panel explores the importance of communication and collaboration among care providers when patients with multiple myeloma who are receiving treatment with bispecific antibodies navigate transitions of care to and from various healthcare settings.

Medical professionals share insights on treatment considerations and patient selection for bispecific antibody therapy and the process for referring patients to other institutions for care.

Sarah Rockwell, PharmD, BCOP, provides an overview of the safety and efficacy data of bispecific antibodies investigated in the MajesTEC-1, MonumenTAL-1, and MagnetisMM-3 trials, providing critical insights into the results of each study.

Ke Ning, MD, discusses the impact of the introduction of bispecific antibodies for treating patients with relapsed or refractory multiple myeloma.
CAR T-cells specifically targeting FCRL5, when integrated with IL-15, may improve cytotoxicity, thereby enhancing the therapeutic potency of these cells.

The vice president of pharmacy operations at American Oncology Network says that pharmacists can contribute by educating patients and offering resources for support and treatment adherence.

Integration of these agents requires supportive care considerations for cytokine release syndrome, neurotoxicity, infection, and antigen-specific toxicities to ensure optimal care of patients with multiple myeloma receiving T-cell–engaging therapies.

Kirollos Hanna, PharmD, BCPS, BCOP, FACCC, addresses some common challenges pharmacists may be facing when looking to operationalize bispecifics at their facilities.

Kirollos Hanna, PharmD, BCPS, BCOP, FACCC, discusses the role of the pharmacist in relation to bispecific care team readiness and patient readiness.
In addition to emerging treatment options, the speakers addressed the importance of individualized decision-making when treating patients with multiple myeloma.
Kirollos Hanna, PharmD, BCPS, BCOP, FACCC, addresses some of the considerations for bispecifics in relation to facility readiness at different sites and among different stakeholders.

The indication is for adult patients with multiple myeloma who are refractory to lenalidomide and have previously received at least 1 line of therapy.

Venetoclax, which is approved for leukemia, blocks a function of the BCL-2 protein and has previously shown efficacy in a small proportion of patients with multiple myeloma.

There are unique complexities when implementing bispecific antibodies, including site of care considerations, monitoring, and management of adverse events.
FDA-approved BiTE therapies for relapsed/refractory multiple myeloma show promising response rates, offering off-the-shelf alternatives to CAR T therapy.
The approval expands the prior indication of idecabtagene vicleucel (Abecma; Bristol Myers Squibb), which will make the drug available to patients in earlier lines.

Unlike conventional monoclonal antibodies, BsAbs possess dual binding domains that target 2 distinct antigens simultaneously.

Panelists summarize key takeaways on using bispecific antibodies for multiple myeloma treatment.
The study authors are hopeful that this finding can help in the development of new drugs or treatments for patients with multiple myeloma.

Carol Ann Huff, MD discusses how the approval of additional bispecific antibodies for multiple myeloma might change the treatment selection process, highlighting considerations for sequencing of agents and combination therapy.

Medical experts describe unmet needs in the treatment of patients with multiple myeloma, describing challenges related to access to care and extramedullary disease.

Michael Singel, PharmD, discusses the role of pharmacists in the operationalization of bispecifics to treat multiple myeloma, including the creation of treatment plans and standard of care order sets.

Michael Singel, PharmD, discusses the logistics of complying with the Risk Evaluation and Mitigation Strategy program for bispecific antibodies at his institution and addresses training and documentation requirements.

The decision to vote in favor of idecabtagene vicleucel comes after positive phase 3 trial results demonstrating its efficacy compared with standard regimens.
Chimeric antigen receptor T-cell therapies were associated with higher incidences, grades of severity, and longer duration of cytokine release syndrome compared with bispecific antibodies.