Tepotinib treats adult patients with metastatic non-small cell lung cancer harboring a mesenchymal epithelial transition exon 14 skipping alteration.

Tepotinib treats adult patients with metastatic non-small cell lung cancer harboring a mesenchymal epithelial transition exon 14 skipping alteration.
Although specialty therapies have the potential to help people live healthier lives, their cost and complexity can create distinct challenges.

Mobocertinib is a kinase inhibitor of the epidermal growth factor receptor (EGFR) that irreversibly binds to and inhibits EGFR exon 20 insertion mutations at lower concentrations than wild type (WT) EGFR.
The results of a study showed that non-Hispanic Black patients treated with immunotherapy had a 15% lower risk of death from non–small cell lung cancer than non-Hispanic white patients.
After 6 to 7 months, just 8 individuals contracted proven symptomatic SARS-CoV-2 infection with rapid favorable evolution, study results show.
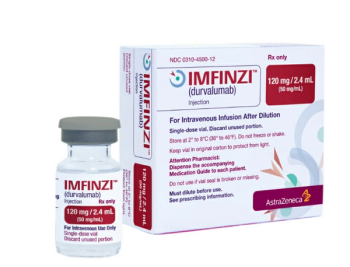
Durvalumab is a human immunoglobulin G1 kappa monoclonal antibody that binds to PD-L1 and blocks the interaction of PD-L1 with PD-1 and CD80.
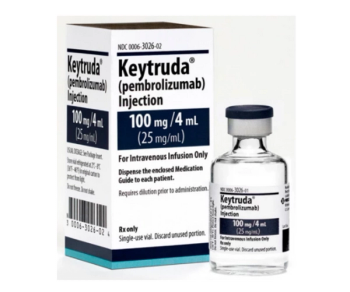
Pembrolizumab (Keytruda) can be used for multiple cancer types, including melanoma, non-small cell lung cancer, and head and neck squamous cell cancer.
The investigators said that the trial data showed potentially clinically meaningful improvements in both progression-free survival and overall survival in pre-specified subgroups of patients based on the baseline inflammatory biomarker, hs-CRP, as well as other biomarker-defined subgroups.

Officials with the FDA have approved Roche’s VENTANA PD-L1 (SP263) Assay as a diagnostic test to evaluate patients with non-small cell lung cancer (NSCLC) who are eligible to receive atezolizumab (Tecentriq, Genentech).
According to Genentech, atezolizumab is the first and only cancer immunotherapy approved for adjuvant treatment of NSCLC.
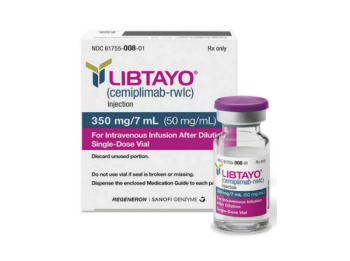
Cemiplimab is a recombinant human immunoglobulin G4 monoclonal antibody that binds to PD-1 and blocks its interaction with PD-L1 and PD-L2.
Between October 2011 and February 2013, UKLS researchers randomized 4055 high-risk participants to either a single invitation to screening with LDCT or to no screening.

Durvalumab in combination with oleclumab reduced the risk of non-small cell lung cancer disease progression or death by 56%, whereas patients receiving durvalumab and monalizumab had a 35% reduction.

Treatment with neoadjuvant cisplatin and pemetrexed plus atezolizumab for pleural mesothelioma found to be safe in trial.
There are 4 essential epidermal growth factor receptor-mutant non-small cell lung cancer subgroups, according to new research results.
The FDA approved Exkivity to treat non-small cell lung cancer along with Thermo Fisher Scientific’s Oncomine DX Target Test, which is intended to be a companion diagnostic for the treatment.

Dr. Jason Liu and his colleagues hoped to assess the use of maintenance durvalumab based on patient and physician characteristics among individuals with stage 3 NSCLC in the United States.

Survey participants saw potential for immunotherapy use in earlier stages of several diseases, including melanoma, lung cancer, and bladder or urothelial cancer.
The regimen with chemotherapy continues to show longer rates at 9 months compared with 4.9 months for chemotherapy and the placebo.
Stereotactic ablative radiotherapy has similar overall and progression-free survival rates.
Individuals who had depression or stress in a hospital in Mexico were also 4.5 and 3.18 times more likely, respectively, to experience delays in treatment.

New artificial intelligence tool can identify tumors at a 97% rate, a year before other methods can diagnose it.

Results from POSEIDON, a randomized open label, phase 3 trial, showed that patients administered 5 cycles of tremelimumab plus durvalumab and chemotherapy over 16 weeks experienced a 23% reduction in the risk of death compared with various chemotherapy options.
Investigators used a new animal model to determine how the stem-like T cell can survive and what it looks like over the course of several months of tumor growth.
The kinase inhibitor is a promising treatment option for patients with MET exon 14 skipping mutation non–small cell lung cancer.