
Researchers question screening guidelines for older women after findings.


Researchers question screening guidelines for older women after findings.

Despite significant advances in cancer treatment, molecular sequencing and enrollment of Black patients in clinical trials are still less than that of White and Asian patients.

Bevacizumab-adcd (Vegzelma) is indicated for the treatment of nonsquamous non–small cell lung cancer; recurrent glioblastoma; metastatic colorectal cancer; persistent, recurrent, or metastatic cervical cancer; metastatic renal cell carcinoma; and epithelial ovarian, fallopian tube, or primary peritoneal cancer.

Overall rates of early-stage cervical cancer in the United States have been falling, but cases with advanced disease are continuing to rise.
Receiving a human papillomavirus vaccine and local surgical treatment could reduce the risk of recurring cervical lesions by 57%.

Studies suggest novel immunotherapy may have efficacy as monotherapy and in combination with an anti-PD-1 immune checkpoint inhibitor.

Bevacizumab-maly (Almysys; mAbxience) is the third biosimilar of bevacizumab (Avastin) approved in the United States.

Numbers have remained below prepandemic levels.

The death rate from cervical cancer has dropped by more than 50% since 1975.

The research team used the National Cancer Institute’s Surveillance, Epidemiology, and End Results database, which provides nationwide information on cancer statistics.

Christopher Mast, MD, vice president of clinical informatics at Epic, discusses research that showed that cancer screening rates in 2021 rebounded from the low rates at the start of the pandemic, but not significantly.

Tisotumab vedotin-tftv is used as antitumor therapy for patients with disease progression during or after chemotherapy.
In the IMPACT trial, women who were positive for high-risk HPV received a follow-up triage test to help determine whether their cervical cells were transforming to cervical pre-cancer.
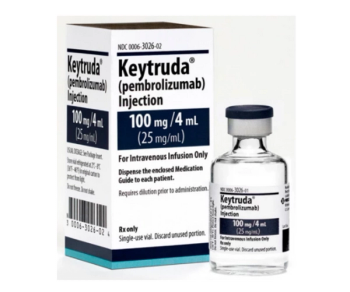
Pembrolizumab (Keytruda) can be used for multiple cancer types, including melanoma, non-small cell lung cancer, and head and neck squamous cell cancer.

The vaccine is used to prevent cervical cancer and other cancers, such as vulvar, vaginal, and oropharyngeal.

Pembrolizumab (Keytruda) approved by FDA in combination with chemotherapy, with or without bevacizumab (Avastin), in patients with persistent, recurrent, or metastatic cervical cancer.
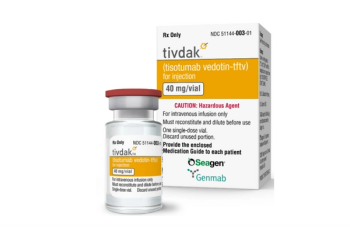
Tisotumab vedotin, is indicated for the treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy.

The Pharmacy Times® Pharmacy Focus podcast provides the latest industry news and information, thought-leader insights, clinical updates, patient counseling tools, and innovative solutions for the everyday practice and business of pharmacy.

Cemiplimab-rwlc is being developed by Regeneron and Sanofi under a global collaboration agreement.

Trial results showed a 24% confirmed objective response rate with a median duration of response of 8.3 months among patients with cervical cancer who received tisotumab vedotin-tftv.
Factors that may have contributed to the screening declines include site closures and temporary pausing of services.

According to the authors of the KEYNOTE-826 trial results, this is the first anti-programmed death receptor-1 (PD-1)/PD-L1 therapy to demonstrate improvements in OS and PFS that were clinically meaningful and statistically significant regardless of PD-L1 status.

Other HPV-associated cancers were found to have increased over the study period, linking this trend to lack of guidelines and other resources.
The trial found positive results in both the overall study population of patients with advanced cervical cancer as well as subgroups with squamous cell carcinoma and adenocarcinoma.

Results from a phase 3 trial investigating the programmed cell death protein 1 inhibitor cemiplimab monotherapy compared to chemotherapy showed an overall survival benefit among patients previously treated with chemotherapy whose cervical cancer is recurrent or metastatic.