
Soliman is ecstatic to celebrate the 4th annual #WomenPharmacistDay and that the pharmacy landscape is continuing to recognize the contributions of women in the field.

Soliman is ecstatic to celebrate the 4th annual #WomenPharmacistDay and that the pharmacy landscape is continuing to recognize the contributions of women in the field.

New legislation in New York state seeks to address flaws in prescription drug distribution system.
Nusinersen (Spinraza) is indicated for the treatment of spinal muscular atrophy (SMA) in pediatric and adult patients.

Respiratory infections can become a problem in muscular dystrophy, therefore immunizations for pneumonia and influenza are vital.

Isatuximab-irfc (Sarclisa; sanofi-aventis) provides patients with another option for treatment and significantly reduces the risk of disease progression or death.

Growing demand for specialty care, shifting prescriber habits, and new reimbursement models have forced stakeholders to adjust practices to optimize patient outcomes.

The objective of this study was to evaluate the safety and immunogenicity of the live attenuated zoster vaccine (ZVL) in patients receiving TNFis, according to the study authors.

Glatiramer acetate modifies immune processes that are believed to be responsible for the pathogenesis of multiple sclerosis.

Prescribers, pharmacists, nurses, lab technicians, and other health care professionals must work as a team to create awareness and understanding of pharmacogenomics integration.

According to a press release from Mirum, maralixibat is the first and only FDA-approved medication to treat ALGS, a rare liver disease that affects 2000 to 2500 children in the United States.
Margetuximab (Margenza) is indicated, in combination with chemotherapy, for the treatment of adult patients with metastatic HER2-positive breast cancer.

Pharmacy Times will be celebrating pharmacists throughout the month of October to recognize their contributions to the health care field.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, explains what the role of the pharmacist is during the treatment of patients with advanced cutaneous T cell lymphoma.

Although specialty pharmacies may be able to offer some of the services that a hub provider does, a hub can standardize patient and provider support across the network.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, addresses the areas that may need further investigation to progress advanced cutaneous T cell lymphoma treatments in the future.

Cemiplimab-rwlc is being developed by Regeneron and Sanofi under a global collaboration agreement.

Durvalumab in combination with oleclumab reduced the risk of non-small cell lung cancer disease progression or death by 56%, whereas patients receiving durvalumab and monalizumab had a 35% reduction.
The combination maintained superior OS and response benefits versus sunitinib in both patients with intermediate- and poor-risk prognostic factors, the primary endpoint population, and across all randomized patients.
Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, explains what research is underway in the advanced cutaneous T cell lymphoma field that she is currently keeping an eye on.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, describes some of the challenges providers face during the treatment of patients with advanced cutaneous T cell lymphoma and how to address them.
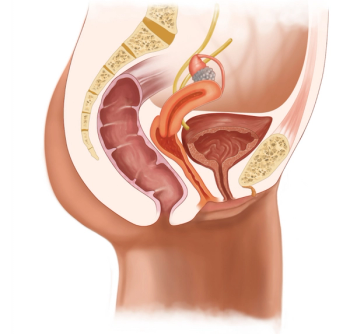
Enfortumab vedotin-ejfv (Seagen; Padcev) is a first-in-class antibody-drug conjugate approved for patients with previously treated locally advanced or metastatic urothelial cancer.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, addresses how the treatment of advanced cutaneous T cell lymphoma has changed in recent years and whether there have been any advancements of note.

ALS results in muscles that are reduced in size (atrophic), weak, and soft, or muscles that are stiff, tight, and spastic.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, provides an overview of key trials that have been conducted in the advanced cutaneous T cell lymphoma space in recent years.
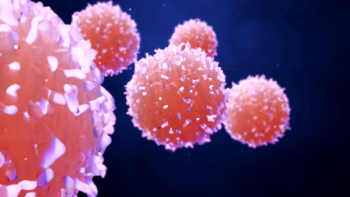
Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, explained whether the current advanced cutaneous T cell lymphoma (CTCL) treatment strategies available are applicable for all patients with advanced CTCL and how patients are selected for treatment.
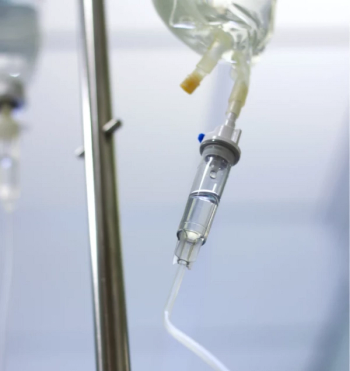
The results of 3 phase 3 trials showed that the drug offered greater proportions of individuals with the condition who met the endpoints of clinical remission and endoscopic response.

Lauren C. Pinter-Brown, MD, FACP, of UCI Health and Chao Family Comprehensive Cancer Center, described what advanced cutaneous T cell lymphoma is in terms of classification and what current treatment approaches are available.
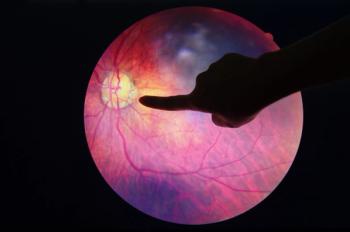
An anti-vascular endothelial growth factor therapy, ranibizumab prevents vision loss in patients with retinal vascular disorders, which can cause irreversible blindness or visual impairments in adults.

REMS are not intended to minimize all harmful effects of a drug, but instead focus on preventing, tracking, and handling a specific serious risk by informing, educating, and/or enforcing measures that reduce the frequency and/or intensity of the event.
Apalutamide (Erleada, Johnson & Johnson) demonstrated a strong prostate-specific antigen response and high adherence rates in patients with non-metastatic castration-resistant prostate cancer.