
Xeljanz can be used for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis.

Xeljanz can be used for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis.

Jameshia Below, PharmD, an assistant professor of pharmacy practice at the University of Louisiana Monroe College of Pharmacy, explains the initial management process for oncologic emergencies, the treatments currently available, and treatments coming in the drug pipeline.

While Bitcoin and Ethereum garner most of the attention in the cryptocurrency space, there are a number of opportunities present for implementing blockchain into global health technology, with perhaps one of the most intuitive being its potential as a platform for health records.
Jennifer Andrews, MD, pediatric hematologist-oncologist at Vanderbilt University Medical Center and medical director of Vanderbilt University Medical Center’s blood bank, discusses how a national blood shortage could affect patient care and outcomes.
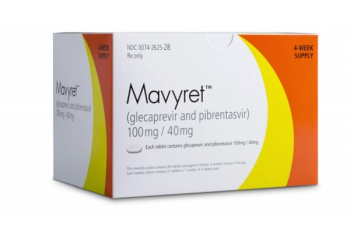
Mavyret is indicated for the treatment of patients with chronic hepatitis C virus without cirrhosis or with compensated cirrhosis.

Payton Nyquvest, CEO and founder of Numinus, said he believes psychedelic medicines will lead to a significant shift in the mental health treatment landscape in the next 3 to 5 years.
Jameshia Below, PharmD, an assistant professor of pharmacy practice at the University of Louisiana Monroe College of Pharmacy, explains what the most common complications from cancer treatment are that can lead to oncologic emergencies and how can they be identified.
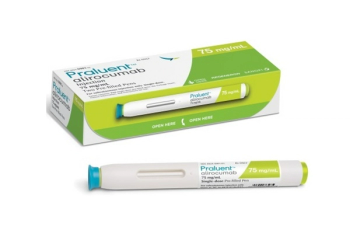
Alirocumab (Praluent) is a PCSK9 inhibitor for the treatment of heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease.

Jennifer Andrews, MD, pediatric hematologist-oncologist at Vanderbilt University Medical Center and medical director of Vanderbilt University Medical Center’s blood bank, discusses what the national blood shortage may mean for health systems.

The Next-Generation Pharmacist Awards national program salutes pharmacy professionals who are defining the industry’s future.

Kelsey Ramsden, CEO of Mind Cure Health, said research into the use of MDMA-assisted therapy could have a huge impact on the treatment of female hypoactive sexual desire disorder.
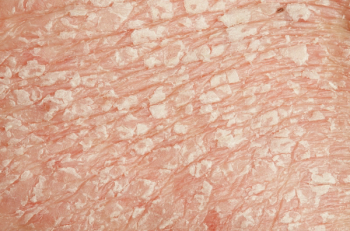
Risankizumab-rzaa treats moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.

Pembrolizumab (Keytruda) approved by FDA in combination with chemotherapy, with or without bevacizumab (Avastin), in patients with persistent, recurrent, or metastatic cervical cancer.
Early intervention with ocrelizumab resulted in a 35% reduction of risk in individuals with RMS who needed a walking aid over 7 and a half years.
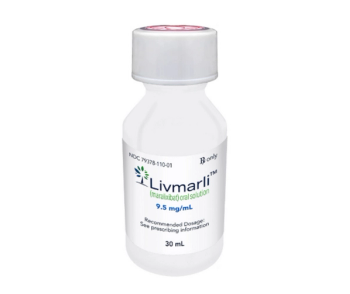
Maralixibat decreases the reabsorption of bile acids from the terminal ileum for the treatment of cholestatic pruritus in patients with Alagille syndrome.

Abemaciclib (Verzenio) approved in combination with endocrine therapy for the adjuvant treatment of adult patients with HR–positive, HER2-negative, node-positive, early breast cancer at high risk of recurrence.

Although October has long been celebrated as National Pharmacy Month, it is only in recent years that female pharmacists have been celebrated for their contributions to the profession and patient care.

Jameshia Below, PharmD, assistant professor of pharmacy practice at the University of Louisiana Monroe College of Pharmacy, explains how oncologic emergencies present in patients, and how each type can be recognized.

Patients receiving sacituzumab govitecan also reported lower symptomatic impact of fatigue, pain, dyspnea, and insomnia.

Fluciclovine F 18 (Axumin), which is a radioactive diagnostic agent for injection that can be used to detect recurring prostate cancer.
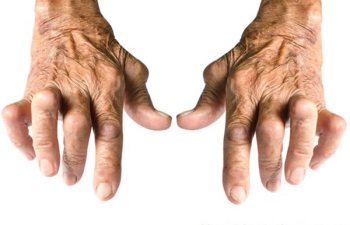
AbbVie announced positive top-line results from a phase 3 trial for the efficacy and safety of upadacitinib for individuals with the rheumatic disease.
The medication, which has been cleared as a treatment for the inflammatory disease of the throat, is under priority review by the agency.

The majority of treatment-related adverse events (TRAEs) were grade 1-2 in severity, and no patients experienced dose-limiting toxicities during the 28 days following initial treatment.

Allostatic load is caused by a number of different stressors, including social isolation, poverty, and racism, many of which are commonly experienced by individuals belonging to racial and ethnic minorities, according to the investigators.

Winners were announced and celebrated at the Next-Generation Pharmacist® of the Year gala on Oct. 8 at Founders Hall in Charlotte, N.C.

There is a real opportunity for technology to alleviate some of the administrative burdens pharmacists face so that they have more time to focus on patient care.
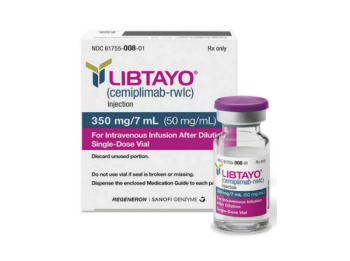
Cemiplimab is a recombinant human immunoglobulin G4 monoclonal antibody that binds to PD-1 and blocks its interaction with PD-L1 and PD-L2.

As practices have gained experience in the post-COVID-19 environment, health care professionals have found innovative ways to work around many of the obstacles the pandemic presents.

Medication adherence, diet, and exercise are each critical components in caring for patients with a chronic illness, but each is difficult to be managed by physicians alone.

Jameshia Below, PharmD, an assistant professor of pharmacy practice at the University of Louisiana Monroe College of Pharmacy, explains what oncologic emergencies are and how they are classified.