
During the COVID-19 pandemic, patients were able to utilize telehealth services to receive care more conveniently, which may reduce the cost of care.

During the COVID-19 pandemic, patients were able to utilize telehealth services to receive care more conveniently, which may reduce the cost of care.

NRTIs TAF and TDF are increasingly being used as preexposure prophylaxis (PrEP) to prevent HIV around the world.

OR-449 is being developed for both adult and pediatric patients with adrenocortical carcinoma, as well as other cancers known to express a high level of steroidogenic factor-1.

Sarah McDonald, cancer survivor and author of The Cancer Channel, discusses her experience battling 2 different unrelated cancers and the value oncology pharmacists could have provided with a greater presence on her care team.

Inequities were also apparent between those who saw and did not see neurologists, analysis shows.
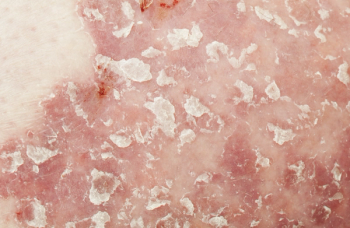
Deucravacitinib is an oral, selective, allosteric tyrosine kinase 2 inhibitor that inhibits cytokine signaling in psoriasis pathogenesis.
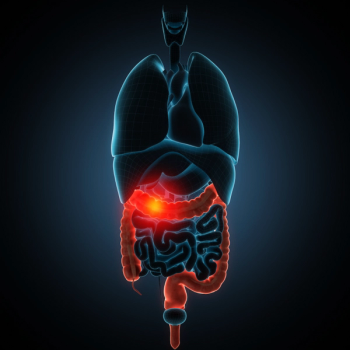
The stool-test is the first-of-its-kind to detect CRC in adults aged 45 to 49 years, with a premarket approval submission planned to be sent to the FDA early in 2023.

Research shows that some unsuccessful switches from reference products to biosimilars may be attributed to patient perceptions of reduced efficacy and safety.

The head of the CAR T cell antibody can recognize and target the growth factor in its tumor microenvironment.
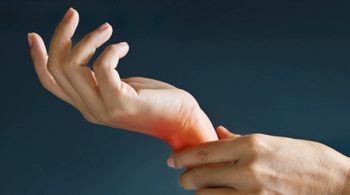
YLB113 (Nepexto) produced significantly lower injection site reactions and injection site erythema compared with etanercept in patients with rheumatoid arthritis.

The analysis found a median progression-free survival of 14.2 months in the ripretinib arm, compared with 1.5 months in the sunitinib arm.

Joseph Araujo, Chief Scientific Officer at Mindset Pharma, sat down to discuss natural vs. synthetic psychedelics.

Patient financial/co-pay assistance is usually a component of providing infusion services and practices need to make sure it’s carefully managed to prevent financial hardship to patients.

Pharmacists report a myriad of reasons for joining the profession, including the connections they can build with patients.
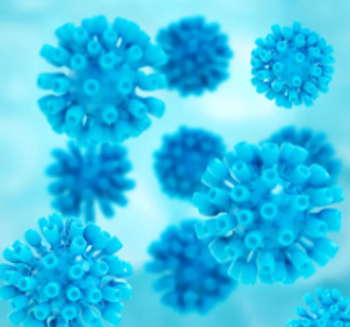
Viral infections can plague patients who have undergone stem cell transplantation.
Analysis indicates that Acadia Pharmaceuticals Inc’s pimavanserin is the only FDA-approved medication for the treatment of delusions and hallucinations associated with PDP.
In the geographical region of a recent study, data showed melanoma cause high mortality rates in relation to incidence.

Digitizing hub processes can create an efficient and effective tech-driven patient access strategy speeding access to lifesaving therapies.

If approved, glofitamab will treat the most aggressive type of non-Hodgkin lymphoma.

Ublituximab-xiiy from TG Therapeutics Inc is the first anti-CD20 monoclonal antibody approved for individuals with RMS that is administered as a 1-hour infusion after the starting dose.

This technology could facilitate equal access to value-based pathways in oncology care and optimize the individual treatment approach.

Big pharma companies have become more measured in their risk taking, with mergers and acquisitions gradually giving way to joint ventures and partnerships.
The FDA granted fidanacogene elaparvovec breakthrough, regenerative medicines advance therapy, and orphan drug designations.

The dual-action cell therapy is designed to eliminate established tumors, train the immune system to eradicate a primary tumor, and prevent recurrence.

The subcutaneous formulation could enhance treatment options by providing high consistency in drug exposure and a convenient administration method.
Clinical trials show that patients with atopic dermatitis experienced improved symptoms with dupilumab starting at 8 weeks, with maximum effect around 12 weeks.

Patient-centric models of medication delivery that provide cost, transparency, convenience, and accessibility solutions are vital for patients to successfully navigate the current health care system.

Stephen Davis, PharmD, senior director of Health System Strategy at Shields, and Erica Diamantides, PharmD, specialty pharmacy manager at UW Medicine, discuss the current guidance and best practice for specialty pharmacy accreditations.

From newsworthy moments to groundbreaking research, these were the most-read articles on Pharmacy Times in 2022.
Participants who survived Hodgkin lymphoma as children showed signs of being biologically older than their peers, with a heightened risk of cognitive problems.