Five-day adjuvant IVIG therapy found to significantly reduce viral load in an immunocompromised patient with severe varicella.

Five-day adjuvant IVIG therapy found to significantly reduce viral load in an immunocompromised patient with severe varicella.

Researchers have identified a channel in nerve cells that may serve as a new approach to targeting Parkinson disease.

Jonathan Ogurchak, PharmD, CSP, sits down with Specialty Pharmacy Times to discuss during the future of data in Specialty Pharmacy at the NASP Annual Meeting in Washington, DC.

Jesse C. Dresser, Esq explains a recent piece of PBM legislation from North Dakota and its implications for specialty pharmacy at the NASP Annual Meeting in Washington, DC.
Top news of the day from across the health care landscape.

Jonathan Ogurchak, PharmD, CSP, sits down with Specialty Pharmacy Times to discuss value-based care and whether or not it poses challenge to data standardization in specialty pharmacy during the NASP Annual Meeting in Washington, DC.
The findings suggest that biologics can be used for those with elderly-onset rheumatoid arthritis (RA) as effectively as for those with young-onset RA.
Top news of the day from across the health care landscape.
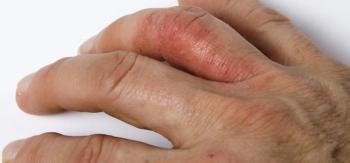
The phase 3b/4 SPIRIT head-to-head study evaluated ixekizumab (Taltz) versus adalimumab (Humira) in psoriatic arthritis.
The FDA approved elexacaftor/tezacaftor/ivacaftor (Trikafta) earlier this month based on the results of a phase 3 clinical trial.

Riluzole oral film (Exservan, Aquestive Therapeutics) has received early-action approval from the FDA for the treatment of amyotrophic lateral sclerosis.

The FDA approved voxelotor (Oxbryta, Global Blood Therapeutics) tablets for the treatment of sickle cell disease in patients 12 years of age and older.
Top news of the day from across the health care landscape.
The case study included a 45-year-old man diagnosed with stage 3 multiple myeloma who was treated with bortezomib and dexamethasone.

If approved, risdiplam (Genentech) would be the first at-home administered medicine for patients living with spinal muscular atrophy.

Jonathan Ogurchak, PharmD, CSP, sits down with Specialty Pharmacy Times to discuss how pharmacy schools can better prepare students for Specialty Pharmacy during the NASP Annual Meeting in Washington, DC.

The study evaluated the safety and efficacy of a live, attenuated zoster vaccine patients receiving tumor necrosis factor inhibitor therapies for various indications.

Top news of the week from Specialty Pharmacy Times.
Top news of the day from across the health care landscape.

The FDA has approved cenobamate tablets (XCOPRI, SK Life Sciences) for treatment of partial-onset seizures in adults.

Under a new international collaboration, the FDA has granted supplemental approval to acalabrutinib (Calquence, AstraZeneca) for the treatment of adults with chronic lymphocytic leukemia or small lymphocytic leukemia.

The 48-week phase 4 study showed that tocilizumab was more effective than rituximab in patients with rheumatoid arthritis with low B cell levels in synovial tissue.

Top news of the day from across the health care landscape.

Jonathan Ogurchak, PharmD, CSP, sits down with Specialty Pharmacy Times to discuss the benefits of attending NASP during the NASP Annual Meeting in Washington, DC.

The FDA has granted approvel to Alnylam’s givosiran (Givlaari) for the treatment of adults with acute hepatic porphyria.
Durvalumab (Imfinzi, AstraZeneca) and tremelimumab plus chemotherapy improved progression-free survival in first-line stage 4 non-small cell lung cancer.

Top news of the day from across the health care landscape.
Researchers in the JADE MONO-1 study investigated whether patients aged 12 years and older with moderate to severe atopic dermatitis would achieve with abrocitinib could achieve clear or almost clear skin with abrocitinib.

Top news of the day from across the health care landscape.

Jesse C. Dresser, Esq explains the current climate of PBM reform at the NASP Annual Meeting in Washington, DC.