
The FDA has issued a warrning letter to JUUL Labs Inc. regarding marketing and advertising practices.

The FDA has issued a warrning letter to JUUL Labs Inc. regarding marketing and advertising practices.

What caused this man to permanently lose his sense of smell?

Misinformation associated with the antivaccination movement has been a driving force contributing to the increase in vaccine preventable diseases, especially amidst the global measles outbreak.

David Pope, PharmD, CDE, chief innovation officer at OmniSYS, and John King, CEO of OmniSYS, discuss how they use data to identify companion vaccines at the point-of-care.

Caring for patients in a rural community is vastly different than in a city, and students at 2 universities got a chance to see the differences firsthand earlier this year.
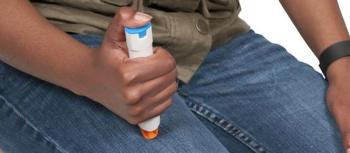
Manufacturers are releasing their own products to meet demand amid the EpiPen shortage.

Jim Kirby, PharmD, BCPS, CDE, and sr. director of pharmacy services for The Kroger Co., discusses his own experiences in providing preventive care services.
SGLT2 inhibitors are indicated to reduce blood glucose by causing the kidneys to remove sugar from the body through the urine.

Johns Hopkins Medical has announced a new Center for Psychedelic and Consciousness Research, funded with $17 million from private donors.
Positive Phase III study results have shown that preventive flu treatment with baloxavir marboxil after exposure to an infected household member reduced the risk of contraction by 86% versus a placebo, according to Roche.

There are currently no FDA-approved targeted therapies to treat metastatic MET exon14 skipping-mutated non-small cell lung cancer (NSCLC), according to the Novartis.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

David Pope, PharmD, CDE, and chief innovation officer of OmniSYS, and John King, CEO of OmniSYS, discuss some opportunities to leverage cash prescriptions to drive revenue in the pharmacy.
The Phase III ETHOS trial for AstraZeneca's budesonide, glycopyrronium and formoterol fumarate, 320/14.4/9.6mcg (Breztri Aerosphere) combination therapy has demonstrated positive results for patients with moderate to severe chronic obstructive pulmonary disease (COPD).

Pharmacists can assist with advising patients who present with quality concerns to use CBD products that provide certificates of analyses, and catch significant drug interactions before they occur.

Jim Kirby, PharmD, BCPS, CDE, and senior director of pharmacy services for The Kroger Co., discusses why preventive care services are necessary in pharmacy practice.
Researchers at Weill Cornell Medicine have found that states requiring prescribers and dispensers to register with and use prescription drug monitoring programs saw significantly fewer opioid prescriptions and reduced opioid-related hospital use, when compared to states with weak drug monitoring program mandates.
These funds are earmarked for the expansion of access to treatment and to support near real-time data on drug overdoses.

Julie Johnson, Dean of the University of Florida College of Pharmacy, on standards of education for pharmacy students around pharmacogenetics and pharmacogenomics.

A federal court has ordered 2 Tennessee-based companies with the same owner to stop disstributing drugs, dietary supplements, and devices until they are found to be in compliance with the Federal Food, Drug, and Cosmetic (FD&C) Act and other requirements.

It is important to have an emergency plan that helps protect pharmacists, pharmacies, and their patients when these catastrophes hit, as well as the aftermath.

Pharmacy Times has announced the addition of the Society of Infectious Diseases Pharmacists (SIDP) to its Strategic Alliance Partnership (SAP) program.

David Pope, PharmD, CDE, chief innovation officer of OmniSYS, and John King, CEO of OmniSYS, discuss how they're tackling medication adherence concerns and how technology can provide a solution. This video was filmed at the 2019 NACDS Total Store Expo.
Community Pharmacy Consultant Bruce Kneeland and Theresa Dickinson, RPh, owner of Melrose Pharmacy in Phoenix, AZ, discusses direct payment options for payers and patients.

The gel product, which was first approved by the FDA in 2013, is intended for cheek augmentation to correct age-related volume deficit in the mid-face in adults over age 21 years.

Jim Kirby, PharmD, BCPS, CDE, and senior director of pharmacy services for The Kroger Co., discusses how chain pharmacies have a unique opportunity in preventive care services.

What is causing the oliguria in this normally healthy young man with a history of acne and a current sinus infection?

Patricia Kienle, BSPHARM, MPA, FASHP, discusses recent revisions to USP 797 and how pharmacists' comments can influence future revisions.

The antivaccination movement has been a driving force in the global measles outbreaks, and dissemination of inaccurate information has contributed to the growing problem.

Julie Johnson, Dean of the University of Florida College of Pharmacy, on the ways pharmacogenetics can be utilized for mental health.