
Congress expected the FDA to draft a memorandum of understanding on interstate distributions of compounded medications that states would be willing to sign, but that’s not what the agency did.

Congress expected the FDA to draft a memorandum of understanding on interstate distributions of compounded medications that states would be willing to sign, but that’s not what the agency did.
The coexistence of hepatitis B virus (HBV) surface antigens and antibodies against are an atypical serological profile for patients with chronic HBV infections.
Teriparatide injection (Forteo) is indicated for the treatment of postmenopausal women with osteoporosis at high risk for fracture or in patients who have failed or are intolerant to other available osteoporosis therapy.

D-Mannose can aid in maintaining urinary and bladder health.

Areas for improvement include the business model, health care team buy-in, patient engagement, and technology.

It is the role of the pharmacist to ensure patients are adequately counseled on the adverse effects and potential harms of codeine.

The topical therapy demonstrated significant improvements in the study, based on IGA success and other endpoints, according to investigators.

PrEP therapy has revolutionized HIV treatment by protecting patients who are high risk for acquiring the virus.

Risankizumab-rzaa (Skyrizi) is the first and only specific interleukin-23 inhibitor approved for the treatment of adults with moderately to severely active Crohn disease.
Contrave is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults.
The role of the pharmacist in sickle cell disease management revolves around appropriate pain management.
The study has met its primary endpoint, with approximately 78.4% of women who continued taking the drug achieving the sustained responder rate through week 76.

Since the COVID-19 pandemic began, more than 10.6 million children have tested positive for the virus in the United States.

The Washington State Pharmacy employs technology for traditional dispensing and in Its compounding lab.
Clinically meaningful results may be a huge step forward in the development of a new therapy for systemic lupus erythematosus.
Over a period of 10 years, substituting antioxidants lutein and zeaxanthin for beta-carotene was found to be more effective at lowering the risk of AMD progression.
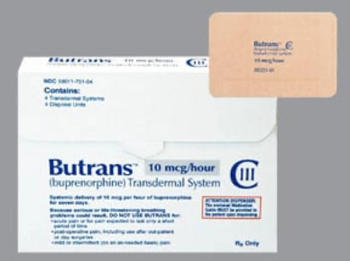
Buprenorphine (Butrans) is a partial opioid agonist indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment for which alternative treatment options are inadequate.

Cough suppressant and antihistamine lowers the frequency and intensity of coughing.

Paxlovid treats COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progression to severe disease.

There are more than 70 cases of monkeypox across 18 states in the United States, as of this week.

Trial court denial of claim Is pursued on appeal based on alleged error by judge when granting summary judgment.
Unlike the 2022 simplification updates for the hepatitis B and pneumococcal vaccine recommendations, ACIP’s recommendations for patient eligibility for zoster vaccination have become more complicated this year.

Lung Support Formula is comprised of natural extracts to help promote lung health.

Data from the phase 2/3 TeenCOVE trial in adolescents met the primary immunogenicity endpoint.

Evidence suggests there are benefits to a more holistic mentoring approach.

Case highlights the need to stop, listen, and investigate when patients or parents express a safety concern.
Lyrica is indicated for neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia, fibromyalgia, spinal cord injury nerve pain, and pain after shingles in adult patients.

Better Nail is an anti-fungal product for fungus under and around the nails.

Angela Livingood, PharmD, MHA, BCGP, CPHQ, diversion specialist at Novant Health, discusses what drug diversion is and why it is important for pharmacists to be aware of it.