
FDA Commissioner Robert Califf said that misinformation is the most common cause of death in the United States.

FDA Commissioner Robert Califf said that misinformation is the most common cause of death in the United States.
When diabetes is uncontrolled, higher percentages of hemoglobin become glycated, which causes elevated HbA1c and glucose levels to rise within the body.

Infection with RSV can take a variety of forms, ranging from a mild upper respiratory tract infection to severe, life-threatening acute respiratory failure.
Psoriasis and end-stage renal disease are believed to share multiple pathogenic pathways, including various cytokines, reactive oxygen species, and psoriasis medications.

Pharmacists are the premier medication experts within health care, who understand more about medication interactions than most physicians and have the unique ability to prevent complications, improve adherence, and save lives.

Teprotumumab is the first fully human IgG1 monoclonal antibody approved by the FDA for the treatment of thyroid eye disease at its source not just reducing the symptoms.
As pharmacists, it’s important to understand and embrace the science and the tools that COVID-19 has brought.

Among nicotine users, there was an approximately 4 beat per minute jump in heart rate after vaping or smoking.

Investigational broadly neutralizing antibody shows promise as potential as a first-in-class treatment option for HIV.
Researchers observed disparities in health outcomes between Black individuals and White individuals due to stroke, diabetes, and coronary heart disease.

Pharmacy leaders and academics have been begging the profession to evolve for decades. It took a pandemic to make it happen.
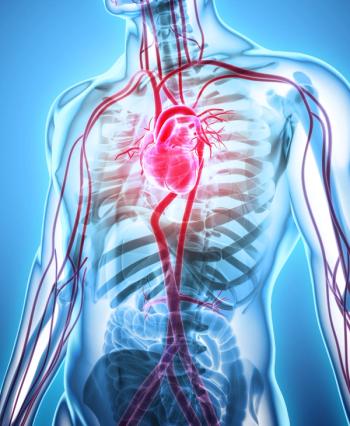
The results showed that these participants were more than 27 times as likely to develop VTE, more than 21.5 times as likely to be diagnosed with heart failure, and 17.5 times as likely to have a stroke.
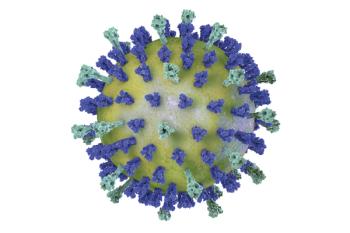
Pre-planned safety reviews performed for the duration of the study found that the investigational vaccine is well-tolerated with no safety concerns for both the vaccinated individuals and their newborns.

Every change in participation offers the opportunity to self-channel to community pharmacies or mail order.

Investing in adequate education and training of new employees up front can save a considerable amount of time and money on the back end.
The drug is a selective, nonsteroidal mineralocorticoid receptor antagonist found to also protect against COVID-19 and pneumonia-related adverse events in patients with diabetes.
The vaccine is suggested to cause more rare cases of thrombosis with thrombocytopenia syndrome than the mRNA-based vaccine, especially among younger individuals.

Tenofovir alafenamide is a nucleoside analog reverse transcriptase inhibitor specifically indicated to treat HBV in adults with compensated liver disease.

From improving access to offering precision care, students are at the forefront of innovation.

Carl Schmid, executive director of the HIV+Hepatitis Policy Institute, discusses how the new once every 2-month injectable PrEP has shown potential to increase adherence among populations with the greatest need.

An ASCP 2022 Annual Meeting session presenter provides an update of 6 new therapeutic agents and their appropriate use in an older adult population.

Problems such as sleep deprivation, excessive sleeping, or inability to sleep regularly may be an important factor in the transition from episodic to chronic migraines.

Herpes zoster can increase the risk of stroke, especially among patients under 40 years of age, for whom the vaccine is not typically recommended.

Technicians are vital members of pharmacy teams and stepped up even more during the COVID-19 pandemic.

Last year in particular, pharmacists played a greater role in patient care with an increased workload as they connected patients, providers, and payers.

Purpose-built assisted reality solutions help streamline processes and maintain regulatory compliance from the laboratory to the factory floor.

Providers working in collaboration with pharmacists saw significantly more patients than providers who worked independently.
New dry eye disease treatment on the horizon.


Covis announced positive topline results for 2 new medications that can treat patients with moderate to severe stable chronic obstructive pulmonary disease.