
An upcoming large-scale clinical trial of the Glucose Everyday Matters program will evaluate its safety and efficacy in managing type 2 diabetes.

An upcoming large-scale clinical trial of the Glucose Everyday Matters program will evaluate its safety and efficacy in managing type 2 diabetes.

The upcoming study seeks to analyze whether immature lungs in infants infected with respiratory syncytial virus increases the likelihood of developing asthma later in life.
Although antiretroviral therapies are the standard of care treatment for those with HIV, the drugs cannot target latent infections; however, venetoclax, a blood cancer treatment, shows promise.

The fixed-dose abacavir/dolutegravir/lamivudine combination is indicated for the once-daily treatment of children weighing at least 6 kg to <25 kg with HIV-1 infection.
Understanding the virus protein’s insertion mechanisms can be useful in creating new COVID-19 vaccine targets.

AI is revolutionizing pharmacy practice by improving medication management, streamlining workflow, and enhancing patient safety and outcomes.

This year's survey features questions focused on compensation, fulfillment, and burnout.

The risk of developing gestational diabetes was similar between both group prenatal care and traditional individual prenatal care, however, group care may benefit some patients as a treatment option.

Tune in to this episode to gain a comprehensive understanding of the evolving role of pharmacists in Michigan's health care system, the potential benefits for patients, and how this paves the way for other states.

With back-to-school season already over and the fall rapidly approaching, health systems are bracing for another season of influenza, COVID-19, and respiratory syncytial virus.
Although the pandemic has negatively influenced the care that patients with cardiovascular conditions receive, the study authors found that there were not significant inequities regarding patients’ race.

Frustrations with the current means of achieving price discrimination have reached a boiling point, but the future remains uncertain.
Pharmacists play a critical role in educating patients on RSV prevention, how to recognize early symptoms, and when to seek medical help.
In a previous trial, DARE-19, investigators reported that the SGLT2 inhibitor dapagliflozin did not benefit patients with COVID-19 who had cardiometabolic risk factors.
Heart health may influence long-term brain health as early as middle age.

Pharmacists can play a crucial role in educating parents and helping remind them about the childhood vaccine schedule, which can be quite complex.
The pharmacy is no longer considered to offer full service (or to be financially sustainable) without a robust vaccination practice.
Study finds that statin use is associated with a 12% reduction in the risk of another stroke.

Results from the VALOR-HCM LTE trial at 56 weeks demonstrated that with longer follow-up, mavacamten continued to reduce patient eligibility for invasive septal reduction therapy.
Patients with lymphoma can be immunocompromised due to their disease, anti-cancer therapy, and concomitant immunosuppressive treatments, which make them more vulnerable to developing a COVID-19 infection.

After 1 year of treatment, semaglutide significantly improved physical function, which can improve quality of life outcomes and risk of death.

SSRI treatment for postnatal depression brought long-term benefits for mothers and their offspring in the following years after childbirth, study findings showed.
A combination of statin therapy and antiretroviral therapy may decrease the naturally higher risk of cardiovascular disease in patients with HIV.

When children got less than the recommended 9 hours of sleep or took more than 30 minutes to fall asleep, there was a strong link to impulsive behaviors later in life.

The seamless integration of artificial intelligence and machine learning has the potential to accelerate research and enhance efficiency in a new era of personalized medicine.
Ferric carboxymaltose was not found to have a significant impact on 6-minute walk distance, which is part of the primary outcome’s hierarchical composite of death and hospitalizations from heart failure.
Any new prices established after negotiations will be effective on January 1, 2026.
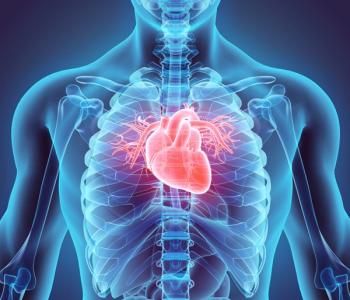
The phase 3 clinical program, CORALreef, consists of 3 trials and is likely the first of its kind for an oral PCSK9 inhibitor, according to the study authors.

The opioid settlement framework could have a significant impact on the future of cannabis distribution and its role in health care.

In this limited series, we speak with experts to understand the new frontier of anti-obesity drugs, as well as concerns and questions that still remain.