
'Nothing succeeds like success' is an old adage in the education world. Today, it is singularly applicable to the independent pharmacy world.

'Nothing succeeds like success' is an old adage in the education world. Today, it is singularly applicable to the independent pharmacy world.

The effect of chemotherapy on functional status can be critical for older adults, as declining function can inhibit the ability to live independently and perform daily activities.

In this video from the National Association of Chain Drug Store's Total Store Expo (NACDS TSE), Anthony Pudlo, PharmD, MBA, Vice President of Professional Affairs at the Iowa Pharmacy Association, talks about technician product verification in Iowa, and how this has benefitted both technicians and pharmacists.

In this interview from the National Association of Chain Drug Store's (NACDS) Total Store Expo, Laura Davis Taylor and Ed King, Co-founders of the High Street Collective, share what they think retail pharmacy will need to look like in the future to stay relevant.

Being accepted into pharmacy school is a great milestone, and the journey was likely filled with hard work and dedication.

Can you solve the pharmaceutical mystery? Each week, a new case study is presented.

The results of the randomized double-blind study, published in the Journal of Drugs in Dermatology, included 120 women with sensitive skin who suffer from clinically diagnosed rosacea, atopic dermatitis/eczema, or cosmetic intolerance
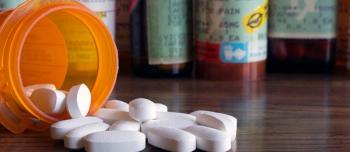
Opioid related overdoses made up 66.4% of the 63,632 overdose deaths in the United States in 2016, prompting the CDC to gather data on fatal opioid overdoses and a prevention plan.

One lot of Montelukast Sodium Tablets is being voluntarily recalled by Camber Pharmaceuticals, due to a safety risk for consumers.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

Officials with the FDA have approved Bayer’s antihemophilic factor [recombinant] PEGylated-aucl, BAY94-9027 (Jivi), for the routine prophylactic treatment of hemophilia A in previously treated adults and adolescents 12 years of age or older

The approvals of Delstrigo and Pifeltro are based on findings from 2 phase 3 clinical trials.


Pharmacists can help eliminate certain barriers to condom use by understanding the range of products available.
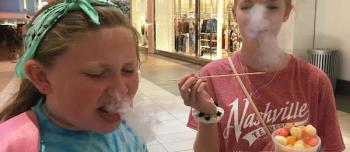
Food products prepared by adding liquid nitrogen at the point of sale, immediately before consumption, have the potential for serious injury, and the FDA is advising consumers to avoid eating, drinking, or handling these products.

The College of Pharmacy at Rosalind Franklin University hosts a competitive summer research program where students can spend the season working alongside RFU researchers making new scientific discoveries.

FDA officials are requiring a warning about this on the prescription information.

One lot of CVS Health 12 Hour Sinus Relief Nasal Mist has been voluntarily recalled. Product Quest Manufacturing expanded the recall to include other products made at the same facility.

On our own Instagram page, Pharmacy Times asked our followers if they had heard about menstrual cups at their pharmacy and if they were interested in learning more.

The pet products included in the recall—sold to both consumers and veterinary professionals—are marketed for a variety of uses, including urinary incontinence, anxiety, arthritis, and digestion relief.

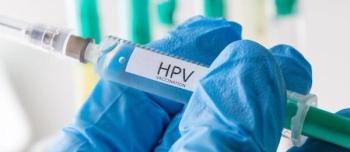
Recent data show that more adolescents are starting and completing the series of HPV vaccinations meant to prevent cervical cancer. Despite the incease in immunizations, some forms of cancers caused by HPV are rising.



Officials with the FDA have approved eravacycline (XERAVA, Tetraphase Pharmaceuticals), a fluorocycline antibacterial within the tetracycline class of antibacterial drugs, for the treatment of complicated intra-abdominal infections (cIAI) in patients aged 18 years and older.

Everything you need to know about WCH from diagnosis, treatment, and a review of the literature.

Have a recruiting and hiring strategy that is unmistakably the best.

One lot of Children’s Advil® Suspension, Bubble Gum Flavored Liquid bottles (4 fluid ounces) is being voluntarily recalled by Pfizer Consumer Healthcare

Accord Healthcare Inc. is voluntarily recalling 1 lot of Hydrochlorothiazide Tablets USP, 12.5 mg, at the consumer level, due to a potential labeling mix-up.
