New results presented at the 2022 ESMO conference reinforce the potential of rucaparib as a first-line maintenance treatment option in a broad population of patients with ovarian cancer.

New results presented at the 2022 ESMO conference reinforce the potential of rucaparib as a first-line maintenance treatment option in a broad population of patients with ovarian cancer.
Data presented at the European Society for Medical Oncology Congress showed that sotorasib increased progression-free survival to 1 year in 25% of patients with KRAS G12C-mutated non-small cell lung cancer.
Fruquintinib reduced the risk of death in patients with advanced metastatic colorectal cancer by 34% and led to longer overall survival than among those given placebo.
Sunvozertinib is an oral, potent EGFR exon20ins inhibitor with wild-type EGFR selectivity.
But the 2021 modifications to the US Preventive Services Task Force recommendations do not eliminate the inequalities, analysis shows.
Study suggests that myeloma patient survival after BCMA-targeted T-cell therapy could be determined by microenvironment factors.
New TROPiCS-02 data for Gilead treatment demonstrates progression-free survival benefit, regardless of HER2 status.

The biosimilar is expected to be launched as a prefilled syringe in early 2023.
Processed meats and products high in added sugars and low in fiber contribute to weight gain and obesity, which is an established risk factor.

Complete recovery of platelet counts after obinutuzumab-based therapy was found to be associated with, or accelerated by, intravenous immunoglobulin.

Final analysis of phase 2 GRIFFIN study from the Johnson & Johnson subsidiary shows the combination demonstrates stringent complete response rate for transplant-eligible patients.

Results from the TOPAZ-1 phase 3 trial showed that the combination reduced risk of death by 20% versus chemotherapy alone.
Analysis shows that over 5 years, the percentage of women without relapse was 89% for those who had hormonal stimulation of the ovaries and 83% for those with ovarian tissue freezing.

DNA and RNA sequencing could provide insight about resistance to chemotherapy in patients suffering from triple-negative breast cancer.
Test can evaluate whether a patient with lymphoma will respond to CAR T-cell therapy, which could lead to better and longer lasting treatment options.

Paul Forsberg, PharmD, BCOP, MHA, director of Pharmacy Services at Minnesota Oncology discusses how pharmacists can remain on top of shifts in the nature of biosimilar payer benefit design.
Specialty pharmacists play critical role in supporting treatment adherence.
Disease management is moving toward holistic care of the patient in the long term.

Atezolizumab meets its primary endpoint of overall survival for patients with resected non-small cell lung cancer across most subgroups.
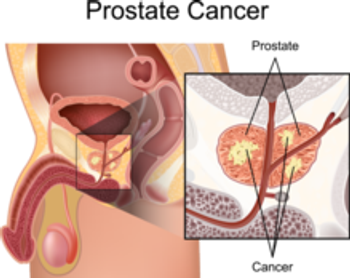
The disease can often be detected and managed early before a patient experiences signs or symptoms.

Pemigatinib is the first targeted therapy to gain FDA approval for the treatment of adult patients with relapsed or refractory myeloid/lymphoid neoplasms with FGFR1 rearrangement.

Risk factors associated with declining health-related quality of life for adult survivors of childhood cancer, such as physical inactivity and chronic health conditions, should be targets of surveillance and intervention.
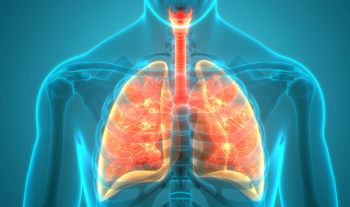
Canakinumab did not meet the primary endpoint of disease-free survival in adult patients with stages II-IIIA and IIIB completely resected non-small cell lung cancer.

In a recent survey, researchers identified rising costs, socioeconomic inequity, and drug trends as major concerns for large employers.

As pharmacists take on new responsibilities, researchers have been studying the effects of pharmacists on interprofessional care teams.

Gene therapies in late-stage development may soon provide hope for a cure.

Overall rates of early-stage cervical cancer in the United States have been falling, but cases with advanced disease are continuing to rise.

Ibrutinib (Imbruvica) is the first therapy to gain FDA approval for younger patients who had no prior treatment options for chronic graft-versus-host disease.

Zynteglo is an autologous hematopoietic stem cell-based gene therapy indicated for the treatment of adult and pediatric patients with β-thalassemia who require regular red blood cell transfusions.

The 4 academic centers will evaluate the role of telehealth in areas such as prevention, screening, diagnosis, treatment, and survivorship.