
Pharmacy Times® will be onsite in Texas providing written and video content for the SABCS, which takes place December 6 to 10.

Pharmacy Times® will be onsite in Texas providing written and video content for the SABCS, which takes place December 6 to 10.

Olutasidenib (Rezlidhia) is indicated for adult patients with relapsed or refractory acute myeloid leukemia with a susceptible IDH1 mutation detected by an FDA-approved test.

A focus on developing positive connectivity among colleagues may help support hematology/oncology pharmacists’ efforts to advocate for roles that optimize their personal and institutional value.

Patient selection is a key factor in the safe and effective administration of advanced pain management modalities.

Data suggest that few US adults are aware that wine can increase the risk of cancer.
The investigational drug is a monoclonal antibody with a high affinity and specificity for cancer-specific plectin, a cell surface protein that correlates with aggressive tumors and poor prognosis.

Data from the fourth interim analysis of the ARC-7 trial shows that a combination of Fc-silent anti-TIGIT and anti-PD-1 molecules are effective for objective response rates in certain lung cancers.

DeepTCR system showed efficacy as a predictive clinical tool and provided information on the biological mechanisms that underlie how patients respond to immunotherapy.
Looking to 2023, there remain significant opportunities on the road ahead to improve patient outcomes and patient-centered oncology treatment and care.

An experimental drug could help scientists understand the development of mantle cell lymphoma and potentially increase overall survival.
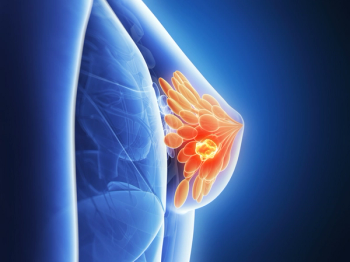
Data to be presented at the 45th Annual San Antonio Breast Cancer Symposium suggest that the novel sequencing test can detect signs of disease recurrence across breast cancer subtypes, improving outcomes.
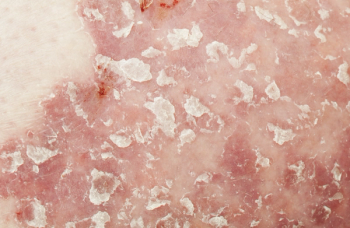
Treatment with immune checkpoint inhibitors have the potential for immune-related adverse events, such as psoriasis.
Zanubrutinib expressed better cardiac safety measures, higher progression-free survival, and lower discontinuation rates in the ALPINE trial compared with compared ibrutinib.

Derazantinib is an investigational oral inhibitor of FGFR1, FGFR2, and FGFR3 in patients with locally advanced or metastatic intrahepatic cholangiocarcinoma.

Study suggests that high levels of nicotinamide riboside could increase the risk of developing triple-negative breast cancer and may cause the cancer to metastasize to the brain.

The trial data demonstrated that pembrolizumab in combination with chemotherapy also had statistically significant and clinically meaningful improvements in PFS and ORR as well.

Patients with lung cancer in an early-intervention lung screening program were found to have a survival rate nearly 20-times longer than patients with a late-stage diagnosis.

Agency’s action date is May 21, 2023, for the first subcutaneous bispecific antibody approved for the treatment of large B-cell lymphoma.

Researchers also noted an increasing rate of seroconversion with COVID-19 booster vaccination series.

New system combines computational and experimental techniques to map evolutionary cancer lineages in their natural habitat of human tissue.
Analysis shows that the therapy is associated with a reduced acute toxicity burden associated with intensity-modulated radiation therapy.

Changes in DNA methylation may lead to accelerated aging in patients with cancer and HIV.
Ponatinib (Iclusig; Takeda) combined with reduced-intensity chemotherapy achieved higher minimal residual disease-negative complete remission rates in adult patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia compared to imatinib.

Sugar…may not always be sweet. Survivors of childhood cancer have a risk of accelerated aging with every 25 grams of sugar consumed, according to researchers.

Investigators also confirmed that FGFR2 fusion–positive tumors have a higher expression of FGFR2 but were not associated with higher expression of FGFR1, FGFR3, or FGFR4.

Mirvetuximab soravtansine-gynx (Elahere; ImmunoGen Inc) approved FRα-positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer.

GDA-501, a novel, genetically modified, nicotinamide natural killer pre-clinical cell therapy.

Belantamab mafodotin-blmf is a first-in-class, anti-B-cell maturation antigen therapy indicated for adults with relapsed or refractory multiple myeloma who have received at least 4 prior therapies.
Under specific circumstances and criteria, they are adjustable to approximate OS in selected populations of Canadian patients who have advanced non–small cell lung cancer.
Tremelimumab-actl (Imjudo) plus durvalumab (Imfinzi) and platinum-based chemotherapy approved for the treatment of adults with metastatic non–small cell lung cancer without sensitizing EGFR mutation or ALK aberrations.