The similarities were shown for both the US formulation and the EU formulation.

The similarities were shown for both the US formulation and the EU formulation.
The blood test offers patients advanced screening methods capable of detecting colorectal cancer in its earlier stages.
Increased levels of absolute lymphocyte count are associated with improved response to BCMA-targeted CAR T-cell therapy.
The new approach could optimize precision medicine and lead to better outcomes across a variety of disease states.
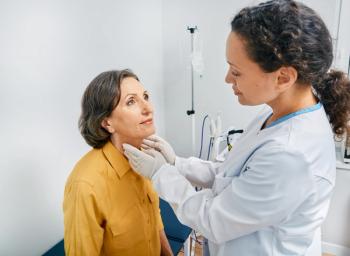
AIC100 could meet an unmet medical need to effectively treat individuals with anaplastic thyroid cancer — the most aggressive form of the disease.

β2-microglobulin may be an effective indicator of coagulation dysfunction in patients with newly diagnosed multiple myeloma (NDMM).
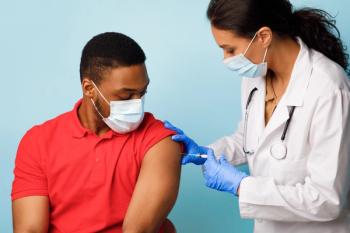
Proper timing and vaccine selection are crucial for optimal immune responses.

Educators should prioritize integrating social determinants of health (SDOH) for the next generation of pharmacists.
There were positive efficacy and safety results for tivozanib monotherapy consistent with previous analyses.
In a study of Swedish patients with mantle cell lymphoma, the participants faced a heightened risk of infection after their diagnosis and associated treatment for the disease.

IMROZ study data show Isa-VRd may be a new standard of care.
NADINA trial results support a new standard of care for these patients.

Prediction model can accurately predict the risk of nerve damage in patients who received taxane-based chemotherapy.

Data presented show significant advancements in treatment options.

The 2024 American Society. ofClinical Oncology (ASCO) Annual Meeting in Chicago, Illinois, highlighted significant advancements across various oncology disciplines.
Treatment of advanced/metastatic urothelial cancer has changed dramatically in recent years. This review discusses the use, risks, and benefits of several novel therapies for this indication.

Roughly one-third of patients with cancer do not achieve adequate pain control.

Extended treatment with high doses of cisplatin-based chemotherapy is associated with severe and progressive hearing loss.

The drug is intended to treat individuals with metastatic or locally advanced melanoma.

Individuals with tattoos were found to have a greater risk of developing malignant lymphoma, with the risk being highest within 2 years of receiving a tattoo.
Researchers found that certain mutations in KLHL6 can lead to an increase in the number of B-cell receptors, resulting in poor outcomes for patients with diffuse large B-cell lymphoma.
ADI-270 is a gamma delta CAR T-cell therapy candidate targeting CD70-positive cancers.
Researchers identify significant proteins involved in the growth and survival of malignant multiple myeloma cells.

Antibody-drug conjugates (ADCs) revolutionize cancer therapy by delivering powerful drugs directly to tumors and minimize damage to healthy cells.

The UK’s Medicine and Healthcare Products Regulatory Agency (MHRA) introduced changes to streamline its drug approval processes.
Omar Nadeem, MD, discusses positive outcomes associated with GPRC5D-targeted CAR T-cell therapy.

The results are promising for patients with relapsed or refractory follicular lymphoma and diffuse large B-cell lymphoma, and the trial is ongoing to assess the efficacy of odronextamab in other subtypes.

FoundationOne Liquid CDx can be used to identify niraparib and abiraterone acetate eligibility in patients with metastatic castration-resistant prostate cancer (mCRPC).
Chimeric antigen receptor (CAR) T-cell therapy can engineer T cells to target malignant cells in multiple myeloma.