
The study is expected to include 460 individuals that will be randomly treated with 12mg of ifinatamab deruxtecan or chemotherapy chosen by the health care provider.

The study is expected to include 460 individuals that will be randomly treated with 12mg of ifinatamab deruxtecan or chemotherapy chosen by the health care provider.
Statins and non-statin cholesterol-lowering agents show potential in reducing the risk of liver cancer.

Dan Schrum discusses the integral role of pharmacy specialists in translating clinical trial results to clinical practice.
The findings may lead to the development of diagnostic and therapeutic targets to prevent worsening disease in patients.
Investigators will examine the combination with pembrolizumab and lenvatinib in patients with HER2-expressing endometrial cancer.

Isatuximab (Sarclisa; Sanofi) in combination with standard-of-care significantly improved progression-free survival in patients with newly diagnosed multiple myeloma.
Dan Schrum highlights the crucial role of lymphodepletion in enhancing the effectiveness of CAR T cell therapy.
Denileukin diftitox-cxdl is the only therapy approved for cutaneous T-cell lymphoma that targets IL-2 receptors in malignant T-cells and Tregs.

Pharmacists can personalize supportive care, educate patients and their caregivers, advocate for patients, and collaborate with other health care workers.
This marks the first FDA-approved systemic therapy for patients with grade 2 astrocytoma or oligodendroglioma with a susceptible isocitrate dehydrogenase-1 (IHD1) or IDH2 mutation.
Replacing carmustine with cisplatin in BEAM ((carmustine, etoposide, cytarabine, and melphalan) conditioning could be more cost-effective for patients.
Acalabrutinib plus venetoclax, with or without obinutuzumab, demonstrated significant improvements in progression-free survival.

A report from SkyQuest Technology highlights the growth in the oncology and insulin spaces, indicating they will contribute to the most growth.

The drug also receives orphan drug designation for the treatment of pancreatic neuroendocrine tumors and a Prescription Drug User Fee Act target action date of April 3, 2025.

The findings highlight the potential for developing more efficacious combination treatments for patients with glioblastoma.
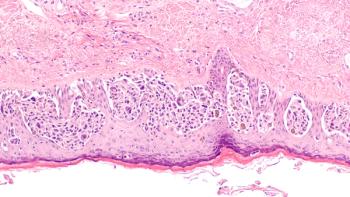
The trial is evaluating the success of BNT111 and cemiplimab in treating unresectable stage III or IV melanoma whose disease had progressed following anti-PD-(L)1-containing treatment.

In the first half of 2024, the FDA approved 23 novel drugs for conditions including alopecia areata, Alzheimer disease, small cell lung cancer, bladder cancer, and more.
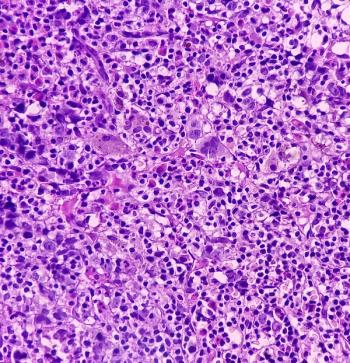
Investigators determine the efficacy and safety of the combination in a 2-phase trial.
Afamitresgene autoleucel (afami-cel) is a novel cell therapy for the treatment of patients with synovial sarcoma.
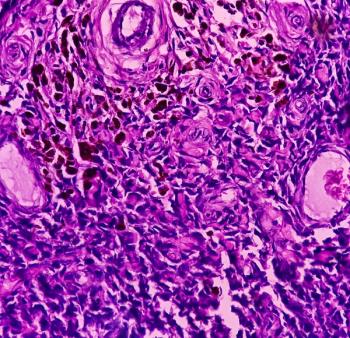
Afami-cel was approved in conjunction with MAGE-A4 IHC 1F9 pharmDx, a diagnostic tool that can identify patients eligible to receive the treatment for synovial sarcoma.

The diagnostic tool can aid the identification of patients with synovial sarcoma who may be eligible for treatment with newly approved afamitresgene autoleucel.
The RUBY trial will continue and analyze the overall population survival after treatment with the drug combination.
The findings provide guidance for clinicians and patients when navigating therapeutic options to establish treatment plans.

Pharmacists' roles in community-based cancer centers are expanding, with a more significant role in managing complex treatment regimens, ensuring comprehensive patient care, navigating drug shortages, and collaborating with multidisciplinary teams to enhance patient outcomes and streamline health care operations.
The drug previously received breakthrough therapy designation for newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia that is in chronic phase.
The decision is based on positive results from the PERSEUS trial.

Jennifer Dunphy, DrPH, MBA, MPH and Nelly Awkar-Lazo, MD, discuss the rise in young-onset cancer, the gut-colon cancer link, and the importance of healthy lifestyle choices for cancer prevention.
The designation is based off positive results from the RAMP 205 trial presented at the ASCO Annual Meeting.
Early identification of monoclonal gammopathy of undetermined significance leads to more efficacious disease monitoring and intervention.
Advancements in treatment include immune checkpoint inhibitors and BRAF/MEK inhibitors, though resistance remains a challenge, with lifileucel recently approved for previously treated metastatic cases.