
Targeted photodynamic therapy has garnered increasing attention… because of its ability to treat tumors without surgery, chemotherapy, or radiation.

Targeted photodynamic therapy has garnered increasing attention… because of its ability to treat tumors without surgery, chemotherapy, or radiation.
This activity is supported by an educational grant from Merck Sharp & Dohme Corp..
A new medication for certain rare blood cancers has now become available through a limited distribution with AllianceRx Walgreens Prime.

Pharmacists should refer patients to a health care provider if any symptoms could be indicative of a GIST.

Challenges and impact of rare diseases are faced by pharmacy stakeholders.

The FDA has approved a liquid biopsy test for all solid tumors with multiple companion diagnostic indications, including for rucaparib tablets.

Targeted radiotherapy amounted to fewer deaths over a 5-year follow-up period among patients with breast cancer.

Researchers have found that gene expression associated with aging was higher in young cancer survivors who are frail and in young patients with cancer following their treatment with chemotherapy.
Patient assistance programs are often the difference between accessing the drug and increasing abandonment rate.

PTCE, the industry-leading continuing pharmacy education provider, becomes sole provider for association’s 3 major meetings through 2021.

In addition to clinical procedures, some support groups for patients with breast cancer were also canceled as a result of the COVID-19 pandemic.
The increasing prevalence of oral oncolytics use, further accelerated by COVID-19, heightens the need for strong patient-pharmacist treatment coordination.

The researchers found that an ILI aimed at weight loss lowered incidence of obesity-related cancers by 16% in adults with overweight or obesity and T2D
Most women with breast cancer receive treatment post surgery, such as chemotherapy, hormone therapy, and radiation. Targeted therapy is useful against cancers that have spread to distant parts of the body.
An online survey found that 44% of breast cancer survivors had care delayed during the COVID-19 pandemic.

Following the expansion of Medicaid coverage under the Affordable Care Act, individuals with low income are less likely to be diagnosed with advanced cancer.
The new analysis of the 23-year follow-up results of the trial found that screening women between 40 and 49 years of age led to a substantial and significant 25% reduction in breast cancer mortality in the first 10 years.

By their training, pharmacists are natural problem solvers, and our education has taught us how to use resources to find an answer and often a solution. The world is waiting for a pharmaceutical solution to coronavirus disease 2019 (COVID-19).
Because triple-negative breast cancer does not express receptors involved in most breast cancers, the investigative therapy could offer a much-needed option.
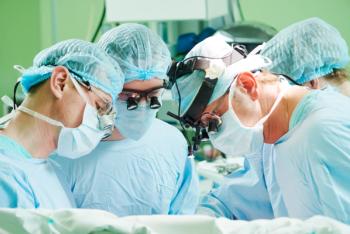
The mortality rates for complex cancer procedures was shown to differ significantly depending on whether the procedure took place at 1-star hospitals or 5-star hospitals.
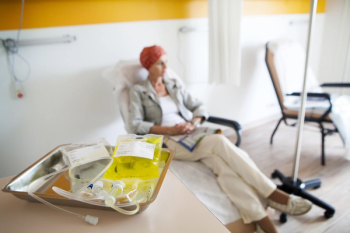
Medical oncologists globally should not discontinue or delay anti-cancer treatment that may potentially impact overall survival during the COVID-19 pandemic.

Due to a robust immune system, young women have a lower response rate to immunotherapy.
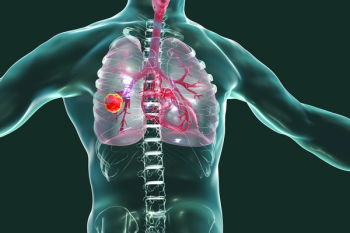
Mortality rates from non-small cell lung cancer have significantly declined in the United States in recent years.
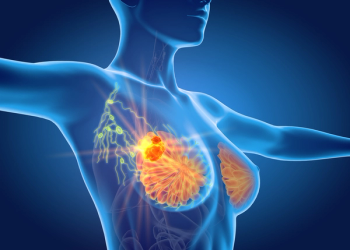
Four new subtypes of cells within triple-negative breast cancer have been uncovered, containing promising new therapeutic targets for the disease.

Patients with relapsed or refractory lymphomas may benefit from CAR-NK cell immunotherapy and Have fewer adverse events.

Cancer survivors’ lean body mass found to be lower than the general population, which was connected with prior abdominal or pelvic radiation.
Like so many other conferences and events, ASCO transitioned to an online format because of the coronavirus disease 2019 (COVID-19) outbreak.
Study shows significant genomic differences between tumors caused by different strains of human papillomavirus.

The combination of sintilimab, pemetrexed, and a platinum-based chemotherapy has shown promise for nonsquamous non-small cell lung cancer.
Human papillomavirus vaccine may reduce the chance of high-grade cervical intraepithelial neoplasis.