PT Staff
Articles by PT Staff


The products include Cold-Eeze Cold Remedy Plus Clod & Flu Symptom Relief, Cellular Nutrition Line, A.M Greens, and Women's Health Multivitamins.

The products include Calcium, Magnesium, Potassium, and Sodium Oxybates Oral Solution, Icatibant Injection, Esomeprazole Magnesium Capsules, and Apremilast Tablets.

The products include Children’s Pain + Fever Gummy Bite, VapoPads Sinu, Clarifying Exfoliant 2% Salicylic Acid, and Menstrual Cramp Relief Cream.





The products include Doxycycline Hyclate Delayed-Release Tablets, Lisdexamfetamine Dimesylate Capsules, Calcium Gluconate in Sodium Chloride Injection, Estradiol Tablets USP

The products include Crovalimab, Dapagliflozin, Secukinumab, Roflumilast

The products include Reserve Sleep Gummies, Menopause Stage Indicator, Organic Ashwagandha Gummies, and Precise Pain Relieving Cream.

Faricimab is the first and only bispecific antibody granted FDA approval for treatment of the eye.

Mirikizumab-mrkz (Omvoh; Eli Lilly and Company) is the first IL-23p19 antagonist approved for adult patients with moderately to severely active ulcerative colitis.
Subcutaneous Lecanemab Found Equally Effective as IV Version in Alzheimer Disease Treatment
A weekly dose of injectable lecanemab-irmb (Leqembi) could allow patients with Alzheimer disease to receive the drug at home instead of visiting an infusion center twice per month.

Nirsevimab-alip (Beyfortus; Sanofi and AstraZeneca) is a monoclonal antibody approved to prevent against respiratory syncytial virus (RSV) lower respiratory tract disease in newborns and infants under 8 months of age born during or entering their first RSV season.
mRNA-1083 is an investigational combination vaccine designed to prevent influenza and COVID-19.

Infliximab-dyyb (Zymfentra; Celltrion USA) is a subcutaneous treatment for the maintenance treatment of adults with moderately to severely active ulcerative colitis and Crohn disease.

FDA Approves Pentavalent Vaccine for Most Common Serogroups That Cause Meningococcal Disease
Penbraya (Pfizer Inc) is the first and only approved pentavalent vaccine that confers protection against the most common meningococcal serogroups—A, B, C, W-135, and Y, in individuals 10 through 25 years of age.
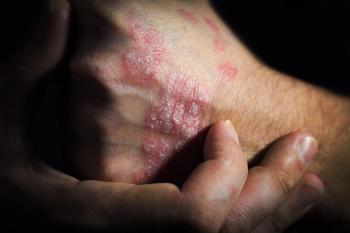
Bimekizumab is a novel, humanized monoclonal IgG1 antibody that potently and selectively neutralizes both IL-7A and IL-17F, which are key cytokines driving inflammatory processes in plaque psoriasis.

Pembrolizumab (Keytruda) gains its sixth approval in non–small cell lung cancer (NSCLC), with the latest indication in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as a monotherapy for the post-surgical adjuvant treatment of patients with resectable NSCLC.

FDA Approves Nivolumab for Completely Resected Stage IIB or Stage IIC Melanoma in Adult, Pediatric Patients
In clinical trials, nivolumab (Opdivo) demonstrated a statistically significant improvement in recurrence-free survival compared to placebo in eligible patients with stages IIB, IIC, III, and stage IV completely resected melanoma.

Encorafenib (Braftovi; Pfizer Inc) and binimetinib (Mektovi; Pfizer Inc) approved for the treatment of patients with metastatic non-small cell lung cancer who harbor a BRAF V600E mutation.

Study Highlights Impact of Preventive Strategies for RSV Hospitalizations in Pediatric Patients
Study shows a a significant burden of hospitalizations associated with respiratory syncytial virus during the 2021-2022 season compared with the prepandemic period.

NVX-CoV2601 vaccine adjuvanted 2023-2024 formula granted EUA for the prevention of COVID-19 in those 12 years of age and older.

Bosutinib (Bosulif) is indicated to treat pediatric patients with Philadelphia chromosome–positive chronic-phase chronic myelogenous leukemia that is newly diagnosed, resistant, or intolerant to previous therapy.


Most patients with myelofibrosis will develop anemia over the course of the disease, with more than 30% discontinuing treatment as a result.

Products include Cyanocobalamin Nasal Spray, Chlorpromazine Hydrochloride Tablets USP, Tiotropium Bromide Inhalation Powder, 18 mcg/ capsule, and Sevelamer Hydrochloride Tablets USP.

Products include Nirsevimab-alip, Remdesivir, Inclisiran, and Lecanemab-irmb.
