
Products include prose skin care, ESPADA 2 plus, hair blend line, and a warm eye mask.

Products include prose skin care, ESPADA 2 plus, hair blend line, and a warm eye mask.

The fixed-dose abacavir/dolutegravir/lamivudine combination is indicated for the once-daily treatment of children weighing at least 6 kg to <25 kg with HIV-1 infection.

Luspatercept-aamt (Reblozyl) approved to treat adults with very low- to intermediate-risk myelodysplastic syndrome who may require regular red blood cell transfusions.


This months generic product news includes azithromycin tablets, minocycline hydrochloride tablets, carboprost tromethanmine injection, and tadalafil tablets.



Elranatamab-bcmm (Elrexfio; Pfizer) is a BCMA-CD3-targeted bispecific antibody approved for adults with relapsed or refractory multiple myeloma who were previously administered at least 4 lines of therapy that included a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.

Niraparib and abiraterone acetate (Akeega; Janssen Pharmaceutical Companies of Johnson & Johnson) is the first-and-only dual action tablet that combines a PARP inhibitor with abiraterone acetate administered with prednisone.

Talquetamab-tgvs (Talvey) is a first-in-class bispecific antibody that binds to GPRC5D and CD3 to induce T cell-mediated killing of GPRC5D-expressing multiple myeloma cells.

Lisaftoclax is a novel, oral, small-molecule, BCL-2 selective inhibitor being evaluated for treatment of relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymphoma.

At a median follow-up of 48.7 months, pembrolizumab monotherapy produced an investigator-assessed objective response rate of 41.5% and a 20.8% complete response rate in patients with relapsed/refractory primary mediastinal large B-cell lymphoma.
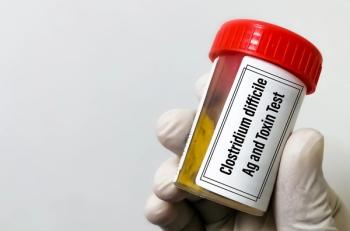
CDI is difficult to contain in health care settings because some patients are unknowing carriers of the toxigenic strains of the infection.
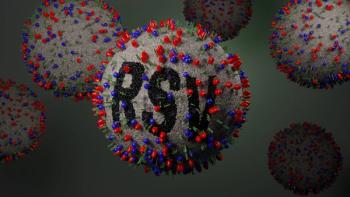
CDC Advisory Committee on Immunization Practices voted 10 to 0 to recommend the routine use of nirsevimab-alip (Beyfortus; Sanofi and AstraZeneca) for the prevention of respiratory syncytial virus lower respiratory tract disease in newborns and infants.

Ervebo was found to be 100% effective after vaccination in preventing the onset of Ebola virus symptoms after more than 10 days.

Dostarlimab-gxly (Jemperli) approved in combination with carboplatin and paclitaxel for the treatment of adults with primary advanced or recurrent endometrial cancer that is mismatch repair deficient.

The approval of RiVive naloxone hydrochloride nasal spray for nonprescription use provides another tool to help quickly reverse the effects of opioid overdose.

A company’s choice of color for branding and logo design may impact how consumers feel about the brand and could influence buyer decision-making.

This months generic product news includes Nitisinone capsules, Prednisolone tablets, Carboprost Tromethamine injections, and Methsuximide capsules.

This months OTC products include brain fuel, SPF 50 mineral sunscreen, women's ensemble, and kids SPF 50 gentle lotion sunscreen.
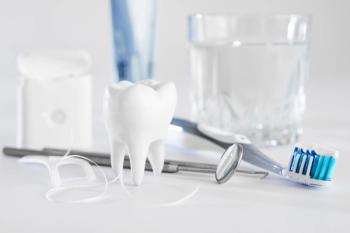
Tooth loss and gum disease may play a role in the health of the brain area that controls thinking and memory.

Irreversible Bruton tyrosine kinase inhibitor produces positive results in patients with relapsed/refractory marginal zone lymphoma.
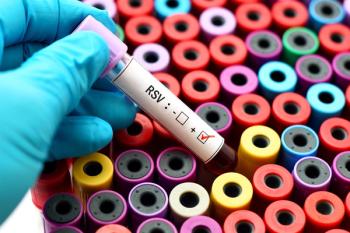
Investigators urge policymakers to establish flexible models that maximize coverage when respiratory syncytial virus activity is high and minimize unnecessary doses when viral activity is low.
Despite injection site adverse effects, the overall benefit-risk balance for pneumococcal vaccination remains favorable.

The updated label for inclisiran (Leqvio) includes patients with comorbidities, such as hypertension and diabetes, who have yet to experience a cardiovascular event.

Drug-induced myopathy is an underdiagnosed adverse effect of treatment with hydroxychloroquine with unclear treatment interventions other than discontinuing the drug.

Includes hair growth supplements and nicotine lozenges.

CDC Advisory Committee on Immunization Practices recommends the use of respiratory syncytial virus (RSV) vaccine, adjuvanted (Arexvy) for adults 60 years of age and older based on findings from the first season of the pivotal AReSVi-006 (Adult Respiratory Syncytial Virus) phase III trial.

Jardiance and Synjardy approved as an addition to diet and exercise to improve blood sugar control in children aged 10 years and older with type 2 diabetes.
A single dose of Arexvy was found effective preventing lower respiratory tract disease caused by respiratory syncytial virus (RSV) in adults 60 years of age and older across 2 full RSV seasons.