
Additionally, birth weight was higher in babies born to mothers who were prescribed glucagon-like peptide-1 (GLP-1) medications either 90 days prior to pregnancy or during the first trimester.

Gillian McGovern is an editor at Pharmacy Times®. She graduated from Rowan University in 2023 with a BA in Writing Arts and concentrations in Publishing & Writing for the Public, Technical & Professional Writing, and Creative Writing.

Additionally, birth weight was higher in babies born to mothers who were prescribed glucagon-like peptide-1 (GLP-1) medications either 90 days prior to pregnancy or during the first trimester.
An increased frequency of immunoglobulin G testing in patients with chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL) was also associated with a lower likelihood of severe infections.

The findings show that the largest gaps are among pediatric patients with persistent asthma.

When treating patients with graft-vs-host disease, pharmacists are often responsible for selecting treatment regimens while navigating other spheres of care.

American Diabetes Association Scientific Sessions speaker Ivan de Araujo discusses how regulation of blood glucose levels and the influence of food preferences can be significant for diabetes care.

HOPA’s DEI Advisory Board Chair discusses how creating safe environments, using correct pronouns, and being willing to talk about patients’ mental and physical needs are significant when providing care to LGBTQIA+ patients.
These findings build off research from March 2023 that demonstrated efficacy and safety of renizgamglogene autogedtemcel in patients with sickle cell disease.

Mice with asthma that were fed bread with Saccharomyces cerevisiae UFMG A-905 yeast showed reduced airway inflammation and decreased IL-5 and IL-13 concentrations, and effects increased when combined with microcapsules.

American Diabetes Association Scientific Sessions speaker Ivan de Araujo emphasizes the importance of the pharmacist’s role in diabetes care and his hopes for the future of care.

Autism spectrum disorder was also evaluated in the analysis, but further research is required to determine a possible association with relative age effect.

Compared with standard of care chemoimmunotherapy, acalabrutinib with bendamustine and rituximab reduced risk of death or disease by approximately 27%.
The findings also show that skin testing positivity for perennial allergens was higher in patients with childhood-onset asthma compared with those with adult-onset asthma.
The indication is for adult patients with primary advanced or recurrent endometrial carcinoma and is the third indication that pembrolizumab has received for this disease state.

With this approval, Yimmugo is the first approved product from the Biotest Next Level production facility.

Creating a supportive environment and staying informed on the latest research can be significant when supporting patients in the LGBTQ+ community, notes Olivia Buckoski, PharmD, AAHIVP, HIVPCP.

The study results also found that exposure to tree canopy area may protect children from asthma development; however, efficacy decreased as weed pollen exposure increased.
The indication is for the improvement of glycemic control in pediatric patients aged 10 and older with type 2 diabetes and comes after positive results from the phase 3 T2NOW clinical trial.
A 1-time infusion of valoctocogene roxaparvovec had demonstrated sustained bleed control and factor VIII expression in patients with hemophilia A.
The default-mode network method, which was analyzed using functional magnetic resonance imaging scans, was approximately 80% accurate when predicting dementia up to 9 years prior to a diagnosis.
Parents and caregivers sometimes have concerns about their children receiving these injections, but researchers and experts assure that the FDA-approved biologic is safe and effective in young populations.
The expanded indication is for the prevention of respiratory syncytial virus-associated lower respiratory tract disease in adults aged 50 through 59 years who are at an increased risk.

Patients with undiagnosed asthma or COPD who received usual care from their primary providers were more likely to visit the hospital or specialists because of respiratory illness.
After allogeneic stem cell transplantation overall survival and relapse-free survival rates in patients with relapsed or refractory acute myeloid leukemia were 52% and 47%, respectively.

The authors noted that these networks have a significant role in intellectual ability, physical coordination, working memory, emotional processing, and overall mental health.

Compared to other cases, the most recent patient reported more flu-like symptoms, including cough, congestion, watery eyes, and sore throat.

Currently, the drug is being evaluated in a phase 1 clinical trial to determine the safety and efficacy in patients.
According to investigators, the primary and key secondary end points were met during the 52-week placebo-controlled period of the trial.
The investigators are optimistic that the data can establish stress-related pathways while revealing potential therapeutic options for patients with major depressive disorder and post-traumatic stress disorder.
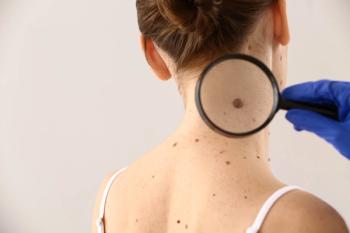
Compared with pembrolizumab alone, the combination treatment showed stronger recurrence-free survival and distant metastasis-free survival in patients with resected high-risk stage III or IV melanoma.

Adults who did not report past 3-day electronic nicotine delivery system use did not have an increased risk of asthma incidence.