
This installment of our series on ADCs and HER2 breast cancer offers key insights about novel agents in clinical trials and management of ADC therapies.

This installment of our series on ADCs and HER2 breast cancer offers key insights about novel agents in clinical trials and management of ADC therapies.
The salvage therapy led to an 80% overall response rate in patients with relapsed/refractory multiple myeloma.

Despite benefits for reducing drug costs, community oncology practices face the unintended consequences of the IRA.
Pharmacists are on the front lines of the outbreak, making them well-positioned to educate and counsel patients with vaccine hesitancy.

A survey showed that 92% of Americans are open to treatments that would slow disease progression.
Douglas Adkins, MD shares key data and findings from the phase 3 KEYNOTE-689 study.

Explore the role of JAK inhibitors in myelofibrosis treatment, focusing on optimizing dosages and managing adverse effects for better patient outcomes.

Biomarker testing transforms non-small cell lung cancer treatment, enhancing outcomes through targeted therapies and next-generation sequencing for better patient care.

Artificial intelligence (AI) models enhance cancer cachexia detection, improving accuracy in predicting muscle atrophy and patient outcomes through advanced biomarker analysis.
Explore the latest advancements in chronic lymphocytic leukemia treatment, focusing on BTK and BCL2 inhibitors, monoclonal antibodies, and patient management strategies.

Pharmacists discuss how drug design impacts drug efficacy and indications for HER2-low and -ultra-low breast cancer.

Hyperglycemia is a common and significant adverse effect of PI3K and AKT inhibitors in hormone receptor-positive (HR+) breast cancer.

Study shows a significant decline in breast cancer mortality across multiple subtypes.
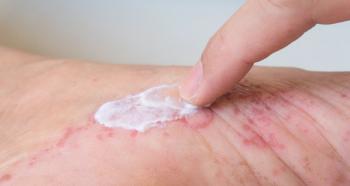
Acneiform rash is a common adverse effect of EGFR-targeting therapies.
Dostarlimab in the neoadjuvant setting yielded a 100% clinical complete response in patients with rectal cancer.
The study found that 4 weeks was the ideal time point to test for cTDNA.
This alternative to IV infusion reduces patient treatment times and eases providers’ operational burdens.
Pyrotinib is a tyrosine kinase inhibitor that is independently developed in China.
The combination yielded superior progression-free (PFS) survival compared with standard of care.
Patients in both arms achieved statistically significant rates of compliance up to 97%.
PAK5 is a protein that drives resistance to trastuzumab emtansine in patients with HER2+ breast cancer.

Thrombosis occurs in approximately 20% of myeloproliferative neoplasms.
Experts attribute the increase of pertussis cases to declining DTaP vaccination rates.
The meaningful results were reported in the DESTINY-Breast09 trial.

Cases have been confirmed in Mexico and Canada.

Real-world data support momelotinib as an effective and well-tolerated treatment for myelofibrosis-related anemia.
Sequencing of antibody-drug conjugates (ADCs) in HER2-mutated breast cancer is crucial for optimizing treatment outcomes.

Undetectable minimal residual disease rates improved with ongoing therapy.
The data showed improvements in overall response rates, progression-free survival, and duration of response.