
Patients who had relapsed or treatment-resistant cancer at baseline, or those who had more severe acute COVID-19 infections, were less likely to develop long COVID-19.

Patients who had relapsed or treatment-resistant cancer at baseline, or those who had more severe acute COVID-19 infections, were less likely to develop long COVID-19.

If approved, the vaccine would be the first immunization for use in pregnant individuals to help protect against the complications of respiratory syncytial virus in infants from birth through 6 months.

Including the ThinPrep Pap Test improves access to the benefits of the human papillomavirus (HPV) assay, which is the only FDA-approved assay that tests for an extended set of HPV types individually.

Pharmacologic approaches can reduce the risk of chronic GVHD.

The approval marks the first and only treatment for geographic atrophy, a leading cause of blindness.

Although naloxone is already available without a prescription in all 50 states, not all pharmacies carry it and those that do must keep it behind the counter.

Study findings confirmed that in asthma, chronic obstructive pulmonary disease, HIV, and influenza, census data differed from the epidemiological data.
Although investigative team is predominantly investigating Priscilicidin for topical applications, they are not ruling out potential oral applications.
Study shows no robust differences in blood pressure, heart rate, lipids, and glucose measurements in children conceived using assisted reproductive technologies.

Trial meets its primary endpoint of progression-free survival regardless of mismatch repair status in patients with stage 3 or 4 or recurrent endometrial carcinoma.
By binding to and inhibiting RANKL, denosumab decreases the production and activity of osteoclasts, resulting in reduced bone loss.

Earlier this week, the FDA Oncologic Drugs Advisory Committee also voted 8 to 5 in support of data for dostarlimab-gxly in rectal cancer.

Individuals with more fast-food options in their communities had 13% higher odds of incident stroke.

In a fact sheet released by the White House, President Joseph Biden called a $35 monthly cap on insulin for all Americans a “commonsense, life-saving protection.”

Men were more likely than women to be prescribed dual blood-thinning therapy following a minor stroke or transient ischemic attack.
Early treatment of poor oral health could lead to brain health benefits.
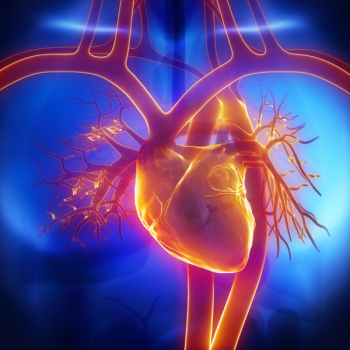
Patients with sepsis were 27% more likely to die, 38% more likely to be rehospitalized for any cause, and 43% more likely to return to the hospital specifically for cardiovascular causes.

Patients with triple-negative breast cancer treated with veliparib had statistically significantly longer survival than those given placebo.

In a clinical trial program, 92% of health care providers, patients, and caregivers were able to successfully administer tezepelumab-ekko both in the clinic and at home for severe asthma.

Belimumab was the first and only approved biologic for active systemic lupus erythematosus and lupus nephritis in more than 50 years, including for pediatric patients.
Study findings mark the first evidence of improved health-related quality of life scores following treatment with an investigational microbiome agent in patients with recurrent Clostridioides difficile.
Peripheral artery disease is more common in Black patients than any other racial or ethnic group, but its potential association with negative social determinants of health is not known.

IQVIA report states that appropriate reimbursement for all health care professionals who provide immunization services would encourage their ongoing provision of vaccines.

CVS and Walgreens have both recently announced that they are seeking FDA certification to use the mail to distribute mifepristone, the first pill used in a 2-drug abortion.
Researchers found a 27% heightened risk of cardiovascular disease for individuals with celiac disease compared with those who didn’t have the condition.

The trial is the first randomized phase 3 study evaluating the efficacy and safety of ciltacabtagene autoleucel patients with relapsed and lenalidomide-refractory multiple myeloma.

The mRNA-1345 vaccine candidate was previously granted Fast Track designation by the FDA in August 2021.

The agency also denied 3 citizen petitions asking for CBD products to be marketed as dietary supplements.
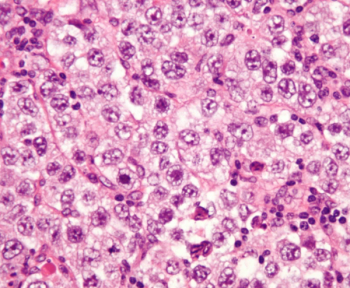
Lisocabtagene maraleucel is a CD19-directed CAR T-cell therapy with a 4-1BB costimulatory domain, which enhances the expansion and persistence of the CAR T cells.

The updated label now includes patients with cold agglutinin disease with or without a history of transfusions.