News
Article
Doxycycline for Post-Exposure Prophylaxis of Sexually Transmitted Infections
Author(s):
In addition to growing rates of STIs, there is concern for antimicrobial resistance to STIs.
The incidence of sexually transmitted infections (STIs), including syphilis, gonorrhea, and chlamydia, continues to rise across the United States. According to data from the US Department of Health and Human Services, the rates of reported cases of primary and secondary syphilis, gonorrhea, and chlamydia rose 71%, 185%, and 19%, respectively, from 2014 to 2018.1 These STI rates continued to increase from 2020 to 2021, but surveillance data during these years were likely impacted by disruptions in STI-related screening because of the COVID-19 pandemic.
Significant disparities in rates of reported STIs persist, likely due to differences in access to quality sexual health care.2 For example, despite non-Hispanic Black persons making up 12% of the US population, 31% of chlamydia, gonorrhea, and primary and secondary syphilis cases were among this patient population in 2021.3
Image credit: James Thew | stock.adobe.com

In addition to growing rates of STIs, there is concern for antimicrobial resistance to STIs. In 2019, the CDC classified drug-resistant gonorrhea as an urgent threat with an estimated 550,000 drug-resistant infections each year.4 New approaches are needed to address these concerns for increasing STI rates.1
One newly proposed method for primary prevention of STIs is the utilization of doxycycline for postexposure prophylaxis (doxy-PEP). When used for STI PEP, doxycycline 200 mg is taken within 72 hours after unprotected sex to prevent STIs.5 Four randomized, controlled trials have evaluated the efficacy and safety of doxy-PEP and are summarized in Table 1.6-10 Two of these studies, in the US and France, found that the use of doxy-PEP was associated with a significant reduction in chlamydia, gonorrhea, and syphilis rates when compared to no PEP.6,10 In contrast, the IPERGAY study found only a reduction in chlamydia and syphilis with the use of doxy-PEP among men and transgender women who have sex with men and are using HIV pre-exposure prophylaxis (PrEP). The non-significant difference in gonorrhea occurrence for the doxy-PEP group was partially attributed to the high prevalence of N. gonorrhoeae strains resistant to tetracyclines in France.9 Only one study evaluated the efficacy of doxy-PEP in cisgender women and found no reduction in bacterial STI rates. Self-reported adherence rates in this study found that 78% of women reported taking doxy-PEP at least as many days as they had sex. However, only 44% of patients in the doxy-PEP group had detectable doxycycline in hair studies, so adherence may have been a reason for the lack of efficacy in this study.7,8,11 None of these trials reported significant adverse events attributable to doxy-PEP.6,8-10
Table: Review of doxy-PEP randomized, controlled trials6-10
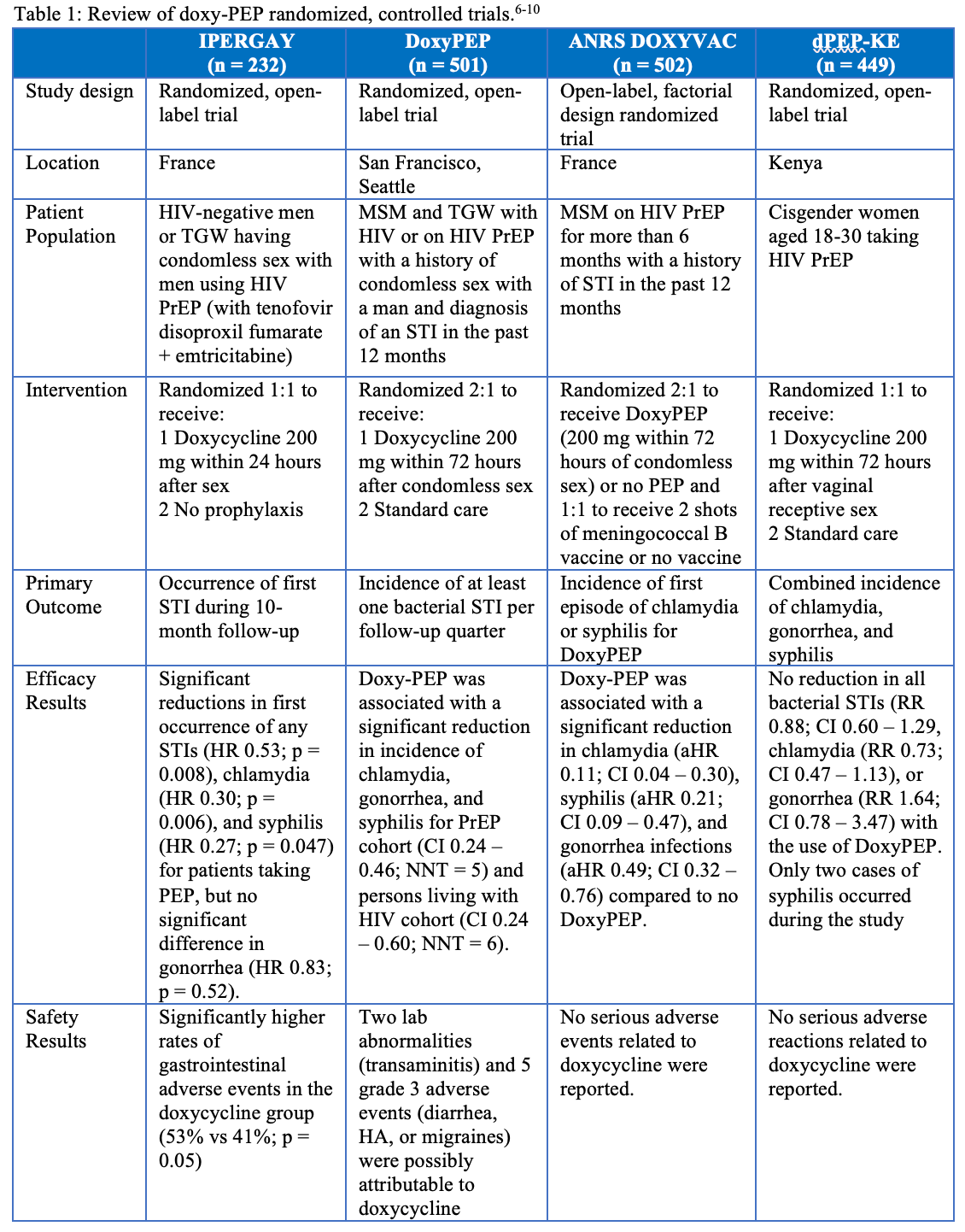
Antimicrobial resistance because of doxy-PEP use is another potential concern that was analyzed in 2 of the studies. In the DoxyPEP study, 27% of N. gonorrhea strains had high-level resistance to tetracyclines (defined as MIC ≥ 2 mcg/mL) at baseline. During follow-up, 38% of N. gonorrhea strains in the doxycycline group were resistant to tetracyclines compared to 12% in the standard of care group. However, these results are limited due to the low rates of susceptibility results (17% of gonorrhea end points). The study found a modestly higher rate of doxycycline resistance to S. aureus among participants with S. aureus colonization in the doxycycline groups than in the standard of care group (16% vs 8%) at 12 months, but the overall percentage with resistance was similar between groups (5% in the doxycycline group vs 4% in the standard-care groups).10
The ARNS DOXYVAC study found that 100% of gonococcal isolates at baseline (n = 7) were resistant to tetracyclines (defined as MIC > 0.5 mg/L for resistance or MIC > 8 mg/L for high-level resistance). During follow-up, 67% and 33% of gonococcal isolates recovered in the doxy-PEP cohort demonstrated resistance and high-level resistance, respectively, compared to 81% and 19% among patients who did not receive doxy-PEP. The study did not find significant trends in development of C. trachomatis strains due to limited phenotype and genotype testing. There were no significant differences between groups for colonization rates of MRSA and ESB-producing E. coli.6 Both of these studies demonstrate that the development of resistance to doxycycline will need to be closely monitored if doxy-PEP is implemented more broadly.
The 2021 CDC STI Guidelines recommended that further studies were needed to determine the benefits of STI PEP as an STI prevention method.5 Since then, additional studies have been completed, which has led to the off-label use of doxy-PEP and the development of doxy-PEP recommendations by some departments of health. The San Francisco Department of Public Health recommends doxy-PEP for cisgender men and transgender women who have had a bacterial STI in the past year and report condomless sex with one or more cisgender males or transgender women in the past year.
Doxycycline 200 mg should be taken ideally within 24 hours but no later than 72 hours after condomless oral, anal, or vaginal sex with no more than one dose in 24 hours. It can be prescribed as doxycycline hyclate delayed release 200 mg or doxycycline hyclate or monohydrate immediate release 100 mg tablets (take 2 tablets simultaneously).12
On October 2, 2023, the CDC requested public comment on a draft of Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention.11 These drafted guidelines provide similar recommendations to the San Francisco Department of Public Health.11-12 These drafted guidelines do not provide a recommendation on the use of doxy-PEP for cisgender women, cisgender heterosexual men, transgender men, or other queer and nonbinary individuals due to insufficient evidence.
Follow-up after prescribing doxy-PEP should include screening for gonorrhea, chlamydia, and syphilis every 3 to 6 months, assessments of doxycycline adverse events, risk reduction counseling and condoms, and providing enough doses of doxycycline until the next follow-up visit after re-assessing the need for doxy-PEP. Public comment on these guidelines will close after 45 days and the proposed guidelines will then be revised as appropriate.11 Doxy-PEP has been shown to be an effective and well-tolerated method to potentially address the STI epidemic, but further studies will be needed to assess the role of doxy-PEP in other patients affected by STIs and its impact on bacterial resistance to doxycycline.
References
1. STI National Strategic Plan Overview. US Department of Health and Human Services. Reviewed July 6, 2022. Accessed November 22, 2023. https://www.hhs.gov/programs/topic-sites/sexually-transmitted-infections/plan-overview/index.html
2. National Overview of STDs, 2021. CDC. Reviewed May 16, 2023. Accessed November 22, 2023. https://www.cdc.gov/std/statistics/2021/overview.htm
3. Black/African American Health. US Department of Health and Human Services Office of Minority Health. Accessed November 22, 2023. https://minorityhealth.hhs.gov/blackafrican-american-health
4. 2019 AR Threats Report. CDC. Reviewed November 23, 2021. Accessed November 22, 2023. https://www.cdc.gov/drugresistance/biggest-threats.html
5. Workowski KA, Bachmann LH, Chan PA, Johnston CM, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1-187. doi:10.15585/mmwr.rr7004a1
6. Molina JM, Bercot B, Assoumou L, Michele AG, et al. ANRS 174 DoxyVac: An open-label randomized trial to prevent STIs in MSM on PrEP. Paper presented at: Conference on Retroviruses and Opportunistic Infections; February 19-22, 2023; Seattle, Washington.
7. Stewart J, Bukusi E, Sesay FA, Oware K, et al. Doxycycline post-exposure prophylaxis for prevention of sexually transmitted infections among Kenyan women using HIV pre-exposure prophylaxis: study protocol for an open-label randomized trial. Trials. 2022;231:495. doi:10.1186/s13063-022-06458-8
8. Stewart J, Oware K, Donnell D, Violette LR, et al. Doxycycline postexposure prophylaxis for prevention of STIs among cisgender women. Paper presented at: Conference on Retroviruses and Opportunistic Infections; February 19-22, 2023; Seattle, Washington.
9. Molina JM, Charreau I, Chidiac C, Pialoux G, et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomized substudy of the ANRS IPERGAY trial. Lancet Infect Dis. 2018;18(3):308-317. doi:10.1016/S1473-3099(17)30725-9
10. Luetkemeyer AF, Donnell D, Dombrowski JC, Cohen S, et al. Postexposure doxycycline to prevent bacterial sexually transmitted infections. New Engl J Med. 2023;388:1296-1306. doi:10.1056/NEJMoa2211934
11. Supplemental material: Guidelines for the use of doxycycline post-exposure prophylaxis for bacterial sexually transmitted infection (STI) prevention. CDC. October 2, 2023. Accessed November 22, 2023. https://www.regulations.gov/document/CDC-2023-0080-0002
12. Doxycycline post-exposure prophylaxis reduces incidence of sexually transmitted infections. San Francisco Department of Public Health Population Health Division. October 20, 2022. Accessed November 23, 2023. https://www.sfcdcp.org/wp-content/uploads/2022/10/Health-Update-Doxycycline-Post-Exposure-Prophylaxis-Reduces-Incidence-of-Sexually-Transmitted-Infections-SFDPH-FINAL-10.20.2022.pdf
Newsletter
Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights.






