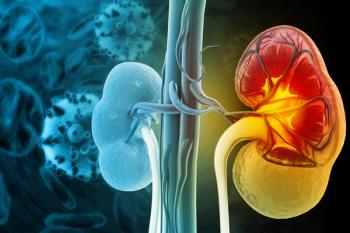
Researchers explore cellular senescence's role in kidney fibrosis, highlighting potential therapies to combat chronic kidney disease progression.

Researchers explore cellular senescence's role in kidney fibrosis, highlighting potential therapies to combat chronic kidney disease progression.
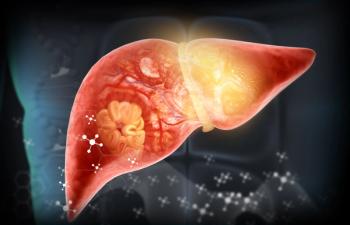
The test offers health care professionals a simple and efficient method of identifying patients with liver fibrosis of varying severity.
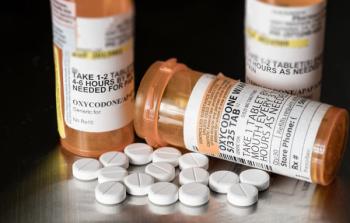
Shore Medical Center enhances patient safety with a pharmacy-driven intravenous to oral opioid conversion protocol, minimizing risks and improving pain management.

The pharmacy is proud to be community focused and patient centered.

CKD is a complication of diabetes. Emerging treatments aim to optimize management and reduce risks. Pharmacists are key in applying new evidence to clinical practice.

Two abstracts from the American Society of Clinical Oncology (ASCO) annual meeting demonstrate that pretreatment DPYD genotyping improves the safety and cost-effectiveness of fluoropyrimidine chemotherapy, with oncology pharmacists playing a pivotal role in implementing and optimizing genotype-guided dosing strategies.
V114, a 15-valent pneumococcal conjugate vaccine (PCV), provided better protection against serotypes 22F and 33F compared with 13-valent PCV (PCV13).
Corrine Stahura, PharmD, and Sophia Gilardone, PharmD, BCOP, discuss recent clinical trial data and evolving guideline recommendations for HER2- and HER3-directed antibody-drug conjugates (ADCs) in non–small cell lung cancer (NSCLC), highlighting differences in efficacy, safety, and patient selection criteria.

Hope Rugo, MD, discusses a multi-institutional real-world analysis showing that rechallenging trastuzumab deruxtecan after grade 1 interstitial lung disease (ILD) is generally safe and effective.
Elranatamab shows promising efficacy and manageable safety in transplant-ineligible newly diagnosed multiple myeloma patients, highlighting its potential as a treatment option.
The investigators suggest that early detection and treatment of chronic kidney disease (CKD) in childhood cancer survivors may decrease late complications and mortality.

The formulation allows for individualized treatment in children with adrenocortical insufficiency.
New research highlights the significant cardiovascular risks associated with RSV, emphasizing the need for vaccination to protect vulnerable populations.
Daratumumab combined with VRd significantly improved outcomes in transplant-ineligible/transplant deferred patients with newly diagnosed multiple myeloma.
Epcoritamab shows impressive long-term remission rates in relapsed large B-cell lymphoma, highlighting its potential as a key treatment option.

FDA approves acoltremon ophthalmic solution, a groundbreaking treatment for dry eye disease, enhancing natural tear production and patient quality of life.

This segment takes a deeper dive into toxicity management—focusing not just on what to monitor, but how institutions are putting these protocols into action in real-world clinical settings.
The FDA approved the MenQuadfi vaccine for young children, enhancing protection against invasive meningococcal disease and its severe complications.

Alice H. Lichtenstein, DSc, emphasizes the importance of whole-food dietary patterns over macronutrient percentages for improving lipid profiles and cardiovascular health.
Pre-treatment DPYD genotyping significantly reduces hospitalizations and costs for patients with cancer, enhancing safety and efficiency in oncology care.
Grace Nguyen, PharmD, BCPS, provides real-world experiences with DPYD genotype-guided fluoropyrimidine dosing, highlighting how pharmacist-led interventions and tailored dose adjustments can help mitigate toxicity risks and optimize treatment in variant carriers.
Corrine Stahura, PharmD; and Sophia Gilardone, PharmD, BCOP, discuss how patient- and treatment-related factors influence the risk of severe toxicity with antibody-drug conjugates (ADCs) in non–small cell lung cancer (NSCLC).

Real change often begins within pharmacies.
Integrated health system specialty pharmacies can improve patient care for non–multiple sclerosis neurologic conditions.

Derek Webb, PharmD, a community pharmacist and Virginia Board of Pharmacy member, provides comprehensive guidance on managing seasonal allergies, distinguishing between symptoms, and exploring treatment options for patients.
A new biosimilar to ustekinumab (Stelara) has been approved by the FDA, further expanding treatment options for patients with immune conditions such as plaque psoriasis and psoriatic arthritis.

Pharmacists have an evolving role in biotechnology.

The expert explored the complex prior authorization process for GLP-1 medications, discussing challenges in documentation and medical necessity.

The data from the phase 3 SEQUOIA study are to be presented at the 2025 ASCO Annual Meeting.