
The significant jump in hospital-owned specialty pharmacies bolsters the belief that health system leaders increasingly understand how these services enable their organizations to enhance patient care and achieve market differentiation.

The significant jump in hospital-owned specialty pharmacies bolsters the belief that health system leaders increasingly understand how these services enable their organizations to enhance patient care and achieve market differentiation.
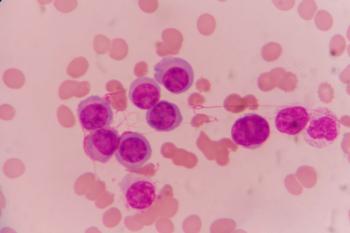
A new study identified pathways that may serve as therapeutic targets for overcoming drug resistance in acute myeloid leukemia.

Pharmacy Universe podcast host Deeb Eid, PharmD talks with Tim Ulbrich, PharmD, Associate Dean of Student Success and Professor of Pharmacy Practice at Northeast Ohio Medical University College of Pharmacy.
An over-the-counter and prescription drug, ranitidine is an H2 (histamine-2) blocker that decreases the amount of acid created by the stomach.

Tenapanor, a novel treatment, is a minimally-absorbed small molecule that acts locally in the gastrointestinal (GI) tract to inhibit the sodium-hydrogen exchanger NHE3.

Nabeel Qureshi, PharmD, expands our understanding of the typical Medical Science Liaison role by taking a look at his position in managed markets in an interview with Matt Paterini of The Nontraditional Pharmacist podcast.
Top news of the week from Specialty Pharmacy Times.

Study finds association between obesity and a greater burden of symptoms and distress among patients with breast or prostate cancer.



As an increasing number of youth are using e-cigarette products, the FDA has announced a federal plan intended to clear the market of unauthorized, nontobacco-flavored e-cigarette products.
Puma’s supplemental New Drug Application is seeking approval for the use neratinib (Nerlynx) plus capecitabine as a third-line treatment in patients with human epidermal growth receptor 2-positive metastatic breast cancer.

The glucagon injection is designed to reduce the steps and time needed to prepare and administer medicine for severe hypoglycemia.
New updates to immunization guidelines in multiple sclerosis outline recommendations on vaccination decisions for patients.

London Mayor Sadiq Khan has called for broader availability of pre-exposure prophylaxis drugs in his welcoming speech at the Fast-Track Cities 2019 conference, where leaders from 300 cities around the world will discuss municipal responses to HIV, tuberculosis, and viral hepatitis.

The #ThankAPharmacist campaign has celebrated and honored pharmacists for the last 5 years.

Suicide is the second leading cause of death among 15-29-year-olds, and one person dies every 40 seconds because of it. This tragedy is preventable with the right education and awareness.
After the Oklahoma case ruling, Johnson & Johnson stocks rallied, but the ruling is a warning to industry leaders that change is necessary.

A recent phase 2 clinical trial found MicuRx Pharmaceuticals’ contezolid acefosamil (MRX-4) to be effective in treating acute bacterial skin and skin structure infections (ABSSSI) with dosing regimens that appeared to be safe and well-tolerated.

Dietary interventions that raise levels of high-density lipoprotein, or good cholesterol, may affect fatigue symptoms in multiple sclerosis.
An autopsy-based study examined long-term trends in HIV-related mortality among individuals living with the disease in New York City.

Hurtado Health Center, Busch-Livingston Health Center, and Cook Douglass Health Center will be closing after over a year of discussion, mainly due to budget constraints and a decline in use by the students in most recent years.

The FDA has issued a warrning letter to JUUL Labs Inc. regarding marketing and advertising practices.

What caused this man to permanently lose his sense of smell?
Results from a phase 2 study demonstrated complete tumor responses in patients with metastatic renal cell carcinoma treated with the combination lixadencel and sunitinib.
Novel combination may improve efficacy of immune checkpoint inhibitors in treating non-small cell lung cancer.

Misinformation associated with the antivaccination movement has been a driving force contributing to the increase in vaccine preventable diseases, especially amidst the global measles outbreak.

Caring for patients in a rural community is vastly different than in a city, and students at 2 universities got a chance to see the differences firsthand earlier this year.
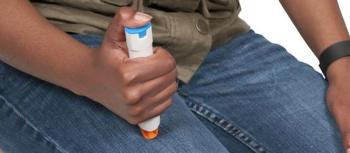
Manufacturers are releasing their own products to meet demand amid the EpiPen shortage.
SGLT2 inhibitors are indicated to reduce blood glucose by causing the kidneys to remove sugar from the body through the urine.