Adverse events included self-limited nausea and diarrhea with no difference between the 2 groups.

Adverse events included self-limited nausea and diarrhea with no difference between the 2 groups.

It turns out that the US Preventive Services Task Force has a recommendation against this supplementation.

Today, we’re celebrating the work of Linda Dumas, PharmD, a pharmacist and pharmacy manager at Publix in St. Augustine, Florida.

The NASP Challenge is an unique opportunity to identify innovative ideas and creative solutions focused on enhancing the specialty pharmacy patient jouney.
Sanofi Pasteur, the vaccines global business unit of Sanofi, is leveraging previous development work for a SARS vaccine as part of a goal to unlock a fast path forward for developing a COVID-19 vaccine.

Going through rotations, students should focus on creating meaningful relationships, and always putting their best foot forward.

Before COVID-19, the health care industry was largely headed toward a value-based system that was shifting to focus on social determinants of health, according to Bruce Japsen, at the PQA 2020 Annual Meeting.
The authors found that telehealth services were facilitated by patients being able to easily share information with providers from equipment that managed CGM for diabetic ketosis and hyperglycemia.

Today, we’re celebrating Keri Bates, PharmD, who works at Rocky Ridge Drug Company in Alabama.

The results will help the rare disease community shed light on the needs of people with rare diseases during the COVID-19 pandemic and other potential health crises, in addition to informing future research efforts.

The study aims to perform a medication risk stratification using drug claims data and to simulate the impact of the addition of various repurposed drugs on medication risk shortages in elderly people.

Top news of the week from Pharmacy Times® surrounding the novel coronavirus.

Which animal has helped treat diabetes?
Staying up-to-date on immunizations is crucial to prevent outbreaks of vaccine-preventable diseases.
Medication therapy problem category framework is the tool used to collect and evaluate medication-related issues
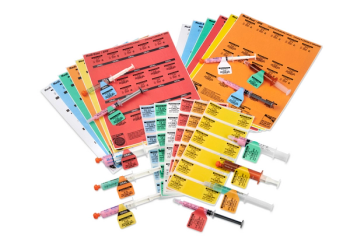
EPS has introduced 5 new colors of Direct Thermal Butterfly Labels to call attention to medication requiring special handling.
The impact of COVID-19 on the health plan enterprise appears to be practical and functional rather than strategic and financial.

Today, we’re celebrating the work of Jessica Louie, PharmD, APh, BCCCP, founder of Spark Joy in Healthcare.

Survey finds that nearly two-thirds of specialty pharmacists spend more than 15 minutes on the phone to fill 1 prescription, whereas 79% of specialty pharmacists seek additional information from clinicians at least 3 times in an average day.

The statement is one of the first from the AHA to focus on providing evidence-based strategies for parents and caregivers to create a healthy food environment for young children.

Researchers at Northwestern University have discovered a strong connection between severe vitamin D deficiency and mortality rates after studying global data from the coronavirus disease 2019 (COVID-19) pandemic.

Although the average wholesale price of the agents is more than $13,000, the investigators found that 9% of patients had a 0% co-pay throughout the 6-month period.
Despite the rate of HIV infection in adolescents and young adults, a study found that PrEP awareness is limited.

A new report from PrEP4All Collaboration warned that current research efforts aimed at controlling the COVID-19 pandemic are missing an important area of research into PrEP.

Margarita Fedorova, CPhT, joins host Jeremy Sasser and co-host Jessica Langley on the latest episode of OnScript with NHA to discuss the innovative pharmacy technician residency program at Cedars-Sinai Medical Center in Los Angeles, the first program of its kind nationally.

Ovarian cancer is the fifth most common cause of death from cancer among women in the United States, according to a press release.

Technological advances enable pharmacists to evolve their practices, spend more direct time in patient care, and assist patients with self-management of their diseases.
Gregory Leighton, RPh, vice president of clinical solutions and patient access for Asembia, discusses how patient support services and hub providers can be used to focus on an individual’s needs and lifestyle for improving medication adherence.

A community pharmacy-based intervention program was utilized to enhance AET adherence.

42.8% of medical staff treating patients with COVID-19 experienced serious skin injuries from the use of personal protective equipment.