
Pregnancy may help put off the onset of multiple sclerosis symptoms by more than 3 years.

Pregnancy may help put off the onset of multiple sclerosis symptoms by more than 3 years.

This week on Pharmacy Times, there are a number of hot topics that we will be posting throughout the week.

Children with a previous diabetes diagnosis showed lower performances compared with newly diagnosed children, suggesting that the deficits may worsen over time.

Less than 10% of the people who should be taking pre-exposure prophylaxis for HIV are taking the medications.

Using a 3D model, investigators found a soy-based compound was effective in treating bone cancer.
Though more research is needed, reactivation of the varicella-zoster virus may be caused by a decrease in absolute lymphocytes caused by COVID-19.
New drug application been submitted for TLX591-CDx (illumet), a radiopharmaceutical product that targets prostate-specific membrane antigen for the imaging of prostate cancer.

It is especially important to better understand the interactions between COVID-19 and acute respiratory distress syndrome.
Two newly discovered antibodies could help in the development of a universal flu vaccine.

Researchers found the largest decreases for heart attack risk among women, which may be because of greater attention paid to women’s heart health in recent decades.

Insurance companies are currently investigating the option of modifying which medications they will cover for HIV pre-exposure prophylaxis (PrEP) therapy, with broad implications for patient access.

In the 18th century, how was tobacco smoke administered to resuscitate drowning victims?
A new study found that hypertension is a common comorbidity in patients with COVID-19.

Researchers at Duke University have found that the efficacy of a 2-pronged type 2 diabetes (T2D) treatment increases when the drugs are linked by a heat-sensitive tether rather than simply being concurrently administered, according to a press release.
Top news of the week from Pharmacy Times®.
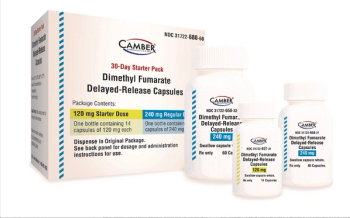
Camber Pharmaceuticals launches Dimethyl Fumarate DR capsules, the generic version of Tecfidera® capsules, which is indicated for the treatment of relapsing forms of multiple sclerosis.

The FDA has granted fast track designation to DKN-01 for the treatment of patients with gastric and gastroesophageal junction adenocarcinoma whose tumors have high DKK1 expression.

An overview of the most common human resources problems that pharmacies face and the proactive steps that pharmacies can take to avoid these problems.

A combination treatment was found to be associated with significantly less weight gain and smaller increases in waist circumference than treatment with olanzapine alone.

The Medication Management Module is a solution for pharmacists to assess each patient’s medication and ensure it is appropriate, effective, and safe given the patient’s comorbidities and medication plan.
A trial will enroll up to 60,000 participants to assess the safety and efficacy of a COVID-19 vaccine candidate that only requires a single dose.
A recent study found that patients on immunosuppressive therapy for common skin and rheumatic diseases, such as psoriasis and rheumatoid arthritis, are not at an increased risk for contracting COVID-19.

Pharmacy Times® interviewed Anne Marie Kondic, PharmD, the executive director of the Community Pharmacy Foundation, on the grant opportunities on the horizon that support the practice of pharmacy in the community setting.
In a comprehensive systematic review that assessed risk factors for HZ infection, researchers found that immunosuppression through HIV/AIDS or malignancy places individuals at a significant risk.
More than half of high-risk patients with resected, stage III BRAF V600-mutated melanoma who were treated with dabrafenib and trametinib were alive and relapse-free at 5 years.

Lactic acid, citric acid, and potassium bitartrate is a prescription combination, non-hormonal contraceptive gel found to be 86.3% effective with typical use.

In recent years, many improvements have been made in the continuous glucose monitoring arena for individuals with diabetes, and there are more products on the horizon.

New refining process helps to minimize odor and taste from fish oil supplements.

NACDS and NASPA have been encouraging state legislators to promote the critical public health message of the importance of flu vaccination this year.
Dr. Reddy’s Laboratories announced the launch of fulvestrant injection, 250 mg/5 mL per single-dose syringe, a therapeutic equivalent generic version of Faslodex (fulvestrant).