
Despite identical HDL cholesterol levels, study participants who had experienced a cardiovascular event showed lower anti-inflammatory activity in their HDL particles compared to the healthy cohort.

Despite identical HDL cholesterol levels, study participants who had experienced a cardiovascular event showed lower anti-inflammatory activity in their HDL particles compared to the healthy cohort.
The duration for body temperature normalization, oxygen saturation, and mechanical ventilation were significantly shorter for patients who received IVIG in addition to standard of care (SOC) when compared to those only receiving SOC.

The Pharmacy Times® Pharmacy Focus podcast provides the latest industry news and information, thought-leader insights, clinical updates, patient counseling tools, and innovative solutions for the everyday practice and business of pharmacy.
Before the advent of modern anesthesia, humans tried multiple avenues for rendering people unconscious before surgeries, with varying results.


Patient interactions became vastly different during the pandemic, with questions surrounding how to coordinate contact and how to best provide pharmacy services virtually, including counseling, fielding drug information questions, medication reconciliation, and providing home medication sheets to families
Olaparib and niraparib both show promise in the maintenance treatment of ovarian cancer, though they may each be appropriate for different patient populations.

Technology is key in supporting and freeing up time for pharmacists to meet vaccination efforts and increased demands in providing community care.
Pharmacokinetic differences in pediatric patients should be considered when administering direct oral anticoagulants, as should potential administration difficulties when pellets or oral solutions are not available.

Chicago Tribune investigation on pharmacists’ time and workload sparks new regulations.
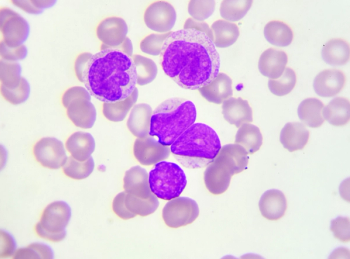
Selection of any BTK inhibitors for mantle cell lymphoma should consider factors such as cardiovascular or bleeding risks, predisposition for gastrointestinal adverse effects, and potential medication nonadherence.

CLL is the most common leukemia in adult patients in the western world, with 21,040 estimated new cases in 2020.
For unresectable gastrointestinal stromal tumors, the introduction of imatinib revolutionized treatment.
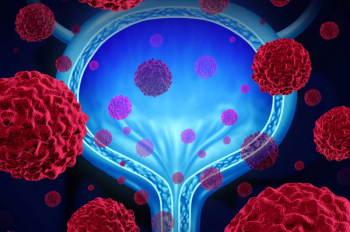
Urothelial carcinoma is the sixth most common cancer diagnosis in the United States, with an estimated 81,400 new diagnoses in 2020 and 17,980 deaths.
Scripta surveyed 372 respondents between 18 and 60 years of age about American attitudes and behaviors around prescription drug pricing.

Second-generation antihistamines are safer and just as affordable and as effective as first-generation antihistamines, according to the study authors.
This study, published in Molecular Psychiatry, builds off of previous research into biomarkers for tracking suicidality, post-traumatic stress disorder, and Alzheimer disease, according to the authors.

Ependymoma is a rare type of tumor that develops in the brain or spinal cord and can occur at any age, but occurs most frequently in young children.
Oral chemotherapies can be effective treatments for children with low-grade gliomas, although clinicians should consider available formulations and administration schedules.
Home infusion within the larger ambulatory infusion setting poses some specific advantages and disadvantages in relation to the other location options available.
In each trial, overall response rate and median progression-free survival in the novel treatment arm showed significant improvement over the sunitinib treatment arm in patients with renal cell carcinoma.

Lakesha M. Butler, PharmD, BCPS, diversity and inclusion coordinator and clinical professor at Southern Illinois University Edwardsville, discussed causes of health inequity and how pharmacists can prevent and mitigate them to improve patient outcomes.
A drug recycling program can help increase patient access and minimize waste of expensive oral cancer medications.
Promising investigational oncology drugs include oral taxanes, small molecule drugs, and immunotherapies.

Common factors that contributed to reported increased dissatisfaction at work include role conflict, quantity of work, workflow disruptions, organizational culture, and leadership support.

Even while therapeutically anticoagulated, patients with cancer have risk factors for recurring venous thromboembolism and bleeding, according to a session at the Hematology/Oncology Pharmacy Association 2021 Virtual Annual Conference.

Research is the first to link chronic sinus inflammation with a neurobiological change.

Heart failure and stroke are rising among individuals below 40 years of age, with links between obesity and low fitness in the late teen years and these early diagnoses.

Errors related to extended-release opioids and COVID-19 vaccines warrant attention and should be a priority.

Sacituzumab govitecan (Trodelvy) approved for patients with locally advanced or metastatic urothelial cancer who previously received a platinum-containing chemotherapy and either a PD-1 or PD-L1 inhibitor.