Remnant cholesterol, a novel lipoprotein marker linked to elevated triglycerides, is associated with increased risk of progression and development of chronic kidney disease.

Remnant cholesterol, a novel lipoprotein marker linked to elevated triglycerides, is associated with increased risk of progression and development of chronic kidney disease.
With robust pipelines, cell therapy remains the major driver for drug development.
Following robust progression-free survival indications in the phase 3 inMIND trial, the FDA granted approval to tafasitamab-cxix in combination with standard-of-care lenalidomide and rituximab for patients with relapsed or refractory follicular lymphoma.

Diet influences blood pressure, cholesterol levels, and overall heart function.

In today’s rapidly evolving health care landscape, pharmacists are playing an increasingly vital role in patient care—but many policymakers remain unaware of the full scope of their expertise and contributions.
The emergence of biomarkers plays a critical role in the growing success of treatment.

Tim Mok, PharmD, BCOP, BCPS, discusses emerging advancements in treatment of leukemia and lymphoma.
New research highlights the significant health risks of respiratory syncytial virus (RSV) in older adults, emphasizing the need for effective vaccines and public health strategies.

In the first half of 2025, the FDA approved 7 novel drugs for oncologic conditions, including for non-small cell lung cancer; ovarian cancer; and metastatic, hormone receptor-positive, human epidermal growth factor 2-negative breast cancer.
When infected with SARS-CoV-2, the virus that causes COVID-19, higher levels of proinflammatory cytokines are released in men compared with women, leading to more severe disease.
Lenacapavir becomes the first and only twice-yearly option for HIV prevention.


Combining omega-3 supplements with exercise enhances immune response and alleviates tooth infections, offering new insights into managing apical periodontitis.
This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.
Accurate utility estimates are critical to providing a comprehensive view of quality of life for children impacted by pneumococcal disease.
This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.

Step up and speak out to break down barriers to health care and address social determinants of health.
New research reveals a significant link between type 2 diabetes, obesity, and fatty liver disease.

This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.

The FDA approves garadacimab-gxii, a treatment for hereditary angioedema (HAE), offering convenient monthly self-injections for patients 12 years and older.
This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.

From protecting the 340B Drug Pricing Program to expanding payment pathways through state-level reforms and Medicare Advantage opportunities, Kraus emphasized the critical role pharmacists play in evolving care models.
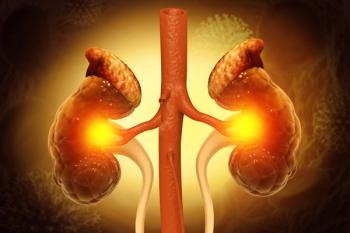
The authors note that the combinatorial principal component analysis (cPCA) may be effective in other complex diseases outside of chronic kidney disease (CKD).
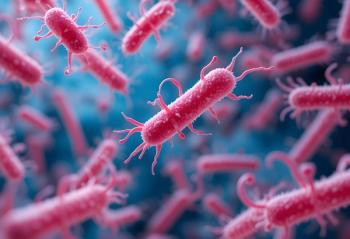
Clostridioides difficile (C difficile) represents a serious public health threat, with pharmacists and advocacy organizations playing critical roles in prevention, education, and management.
This single-center, descriptive, retrospective chart review identified barriers to outpatient chemotherapy use, revealing avoidable inpatient stays and highlighting targets for stewardship interventions.
The opportunity to optimize all medications, not just those that require a prescription, is long overdue.
Eli Lilly is expanding access to tirzepatide, a groundbreaking GLP-1 medication for weight management, offering more affordable options for obesity treatment.

Discover the latest FDA-approved treatments, including Dupixent for chronic urticaria and Valtoco for seizure clusters, enhancing patient care options.

Host Craig Beavers chats with Shannon Finks, PharmD, FCCP, BCPS, BCCP, AHSCP-CHC, president and director of pharmacy services at ZupMed in Memphis, Tennessee.
This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.