Norman Lepor, MD, a cardiologist and clinical investigator in the phase 3 clinical program for inclisiran, discussed the trial results assessing the efficacy and safety of inclisiran for patients with atherosclerotic cardiovascular disease.

Norman Lepor, MD, a cardiologist and clinical investigator in the phase 3 clinical program for inclisiran, discussed the trial results assessing the efficacy and safety of inclisiran for patients with atherosclerotic cardiovascular disease.
Drug shortages, infrastructure and wholesaler challenges will be the backdrops of the pharmacy landscape this year.

Shreejit Nair, senior vice president and life sciences market business lead at CitiusTech, discusses what data interoperability is and what it looks like within a pharmacy context.
Drug manufacturers participating in Medicaid offer lower prices, stretching resources and providing comprehensive services to more patients.

Continuing leadership education can help further develop pharmacy leaders by teaching effective utilization of various power strategies.

While progress is being made to advance the Cures Act, it is essential to ensure pharmacy interoperability given pharmacy’s critical role in health care.

The sBLA is reinforced by results from a randomized phase 3 trial that investigated cemiplimab-rwlc in combination with a physician’s choice of platinum-doublet chemotherapy compared to platinum-doublet chemotherapy alone.

The researchers projected that approximately 14 participants would experience a significant worsening of their walking function, however, only 8 participants had their walking function decline.

The Biden administration announced that it would make 400 million nonsurgical N95 masks for US citizens available free of charge at community health centers and retail pharmacies across the United States.
One viral protein could provide information to deter pneumonia causing the body’s exaggerated inflammatory response, new study results show.

Did trial court judge err when dismissing the lawsuit without direct evidence of plaintiff’s claim?

IMX-110 is a tissue-specific therapeutic designed to accumulate at intended therapeutic sites at 3 to 5 times the rate of conventional therapeutics.
The phase 3 ARASENS trial is the only study prospectively designed to evaluate an androgen receptor inhibitor combined with docetaxel and androgen deprivation therapy in this patient population.

With shifts in health care moving toward AI and automation, issues relating to how these innovations may impact patient safety continue to emerge.
Future studies are necessary to continue evaluating the true benefits of dupilumab in conditions like atopic dermatitis.
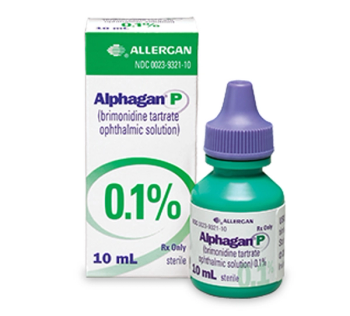
Brimonidine ophthalmic solution (Alphagan P) is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Treatment for geographic tongue includes OTC pay relievers, mouth rinses with anesthetics, antihistamines, corticosteroids, and vitamin B supplements.
Results from multiple studies show that the effectiveness of nirmatrelvir, the active main protease inhibitor of Paxlovid is maintained.

Study results show that unvaccinated pregnant women with the disease are more likely to experience poor childbirth outcomes, even if they did not have severe respiratory problems.
Oral apremilast achieved a clinically meaningful and statistically significant improvement in the primary endpoint of the modified static Physician’s Global Assessment of Genitalia response.

Ending the COVID-19 pandemic requires an understanding of how to manage the inflammatory dysfunction that the virus causes.
Agency green light could help get medication off ASHP drug shortage list by reducing supply issues.
The FDA has approved zanubrutinib (Brukinsa, BeiGene) for the treatment of adults with Waldenström macroglobulinemia (WM).
After varicella zoster virus causes chickenpox, it remains dormant in the central nervous system and can later reactivate to cause shingles

The findings from the phase 3 KEYNOTE-091 trial mark the first positive study for pembrolizumab in adjuvant stage IB-IIIA non-small cell lung cancer.
Emetrol is used to treat an upset stomach and vomiting.
Combigan is comprised of 2 components that reduce elevated intraocular pressure, whether or not associated with glaucoma.

Amy Summers, consultant at Restore Health Consulting, discusses the proposed changed to the USP 795 and 797.

Evaluating the need to define and standardize how quality can be measured in pharmacy.
If glaucoma is recognized early, vision loss can be slowed or prevented.