
Kaléo’s product has received fast-track designation for US military personnel facing this threat on the battlefield.

Kaléo’s product has received fast-track designation for US military personnel facing this threat on the battlefield.

Durable responses mark a significant advancement over chemotherapy, which may have only been effective for 9 to 10 months.
Study findings support the role of tezepelumab-ekko as a first-in-class treatment for a broad population of patients living with severe asthma, irrespective of biomarker levels.
Acceptance is based on CheckMate -816 trial results where the agency granted priority review status to the Bristol Myers Squibb drug and assigned a PDUFA goal date of July 13, 2022.

Investigators at Penn State find that these medications inhibit certain viral enzymes called proteases that are essential for SARS-CoV-2 replication in infected human cells.
COVID-19 vaccines are effective in preventing severe infection resulting in hospitalization, but protection wanes after 6 months.

There are a few options that can help with the current mental health epidemic, although more studies are recommended to perfect these treatments.

New strategies are needed to increase HIV prevention and testing efforts among high-risk communities.
Upadacitinib (Rinvoq) is a Janus kinase (JAK) inhibitor being investigated to treat rheumatoid arthritis, Crohn disease, ulcerative colitis, atopic dermatitis, psoriatic arthritis, axial SpA giant cell arteritis, and Takayasu arteritis

BenGay offers relief from minor arthritis pain, backache, muscle and joint pain.
Michael Abrams, MPH, PhD, senior health researcher at Public Citizen’s Health Research Group, discusses why classification of gabapentin as schedule V might be beneficial.

Jamie Wilkey, PharmD, founder of PGx Consulting Confidence Academy, discussed how pharmacists are utilizing pharmacogenomics for their patients.

While staffing challenges mean health leaders may have to shift priorities and resources, one area where we shouldn’t scale back is in our efforts to mitigate health care drug diversion.

Jakob Jensen, PhD, professor in the Department of Communication at the University of Utah and member of the Huntsman Cancer Institute, discusses how the beliefs and perceptions of cancer among adults living in rural areas compared to those of adults living in urban areas.

Filgrastim-ayow is indicated for the treatment of neutropenia, which is commonly experienced by patients receiving chemotherapy.

Although biologic changes may occur in the body that affect the brain, nonbiologic changes, such as social isolation, can also be a part of it but the reasons are not completely clear, according to the study.

Gauging patients’ satisfaction with pharmacy services provides an opportunity perhaps for improved patient outcomes in certain situations.

Thomasina Morris, RPh, MHA, BCOP, pharmacist at Moffitt Cancer Center, discusses how dosing for rucaparib treatment is assessed to treat patients with ovarian cancer.
At week 4, there was about a 61.6% decrease for individuals with hypercholesterolemia who do not attain low-density lipoprotein cholesterol and/or are intolerant to other lipid-lowering drugs.

During spring to fall 2020 related visits to emergency departments declined compared with a year earlier, study results show.
The agency has given the greenlight to the abbreviated new drug application for the overdose-reversal medication.

The CAR T-cell therapy CYAD-101 was being evaluated in combination with FOLFOX followed by pembrolizumab (Keytruda) in patients with unresectable metastatic colorectal cancer.
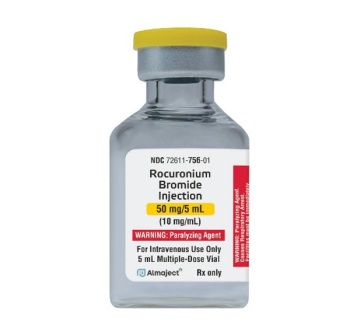
Rocuronium bromide is indicated as an adjunct to general anesthesia and to provide skeletal muscle relaxation during surgery or mechanical ventilation.

Odor-Eaters foot powder removes foot odor and offers possible wetness protection.
Lakyn Husinka, PharmD, discussed new treatment options and pharmacists' role in treating patients with breast cancer.

PrEP involves taking emtricitabine/tenofovir (Truvada or Descovy) to prevent high-risk individuals from contracting HIV.

The researchers aimed to identify the factors that may influence the risk of statin intolerance.

Breadth, intensity of vertical integration keeps growing, leaving pharmacies' future role uncertain.

Erenumab (Aimovig) was the first anti-CGRP agent approved by the FDA for migraine prophylaxis.
Shingles can cause significant pain and, in some rare instances, can be life-threatening.