The drug combination demonstrated a 64.5% confirmed objective response rate in individuals with unresectable locally advanced or metastatic urothelial cancer who are ineligible to receive cisplatin-based chemotherapy.

The drug combination demonstrated a 64.5% confirmed objective response rate in individuals with unresectable locally advanced or metastatic urothelial cancer who are ineligible to receive cisplatin-based chemotherapy.

The FDA is evaluating the use of adagrasib (MRTX849) to treat patients with non-small cell lung cancer harboring a KRAS G12C mutation who have previously received at least 1 systemic therapy.

The first thing is educating these folks, because it's hard for them to understand our challenges if they don’t understand what we do.

This month's featured products include iloperidone tablets, dabigatran etexilate capsules, and more.
They can assess for adherence, identify drug-related problems, monitor pharmacotherapy, and provide education.
No differences were found among placenta health indictors, birth weights, or well-being scores between vaccinated and unvaccinated pregnant women, indicating that COVID-19 vaccination is safe for use in pregnant women.

People who performed 2 to 4 times above the recommended amount of moderate physical activity (300-600 minutes/week) saw an overall 26%-31% lower risk of mortality from all causes.

Get involved, attend their sessions, attend their conferences, understand what they’re saying, make sure that we’re, again, speaking with that one common voice.
Records from 11 million patients shows that non-alcoholic fatty liver disease could increase the risk of developing heart failure by 50%.

Researchers highlight how a lack of access to medications used to treat multiple sclerosis, migraines, and epilepsy, but not proven safe for pregnancy, threatens child-bearing aged women who live with these conditions.
Argatroban is indicated for thrombosis in adult patients with heparin-induced thrombocytopenia and as an anticoagulant in adults patients with or at risk for HIT undergoing percutaneous coronary intervention.

Topical symptomatic relief for resistant skin conditions, including severe boils, hemorrhoids, eczema, cold sores, fungal infections, psoriasis, poison ivy, oak and sumac, and other itchy, painful conditions.
Pirtobrutinib is under investigation in clinical trials in patients with CLL/small lymphocytic lymphoma, mantle cell lymphoma, and non-Hodgkin lymphoma.

The number of medical visits carried out via telehealth grew from 840,000 in 2019 to 52.7 million in 2020.

FDA accepts supplemental Biologics License Application for Hyrimoz HCF, a biosimilar with the same indications as adalimumab (Humira).

SARS-CoV-2 infection was associated with post-COVID-19 conditions for children, with associated risk factors including length of hospitalization, number of acute symptoms, and older age.

You just need to tell your story and be genuine and be real, and it makes such a difference.

Two pharmacist-based deprescribing models reduced the use and prescription of anticholinergics, a high-risk drug in older adults, providing evidence for the use of pharmacists is practitioners of deprescribing.

With the vast number of drugs that can induce photosensitivity, patients taking any of these medications should receive safety counseling, especially during the summer months.

It is important to step back and see value creation for services from a broader and more fundamental perspective than the individual, daily tasks in the pharmacy.
Researchers suggest that the current case definition of monkeypox be broadened to include genital lesions and sores on the mouth or anus as symptoms to prevent misdiagnoses.
Trastuzumab deruxtecan is an engineered HER2-directed antibody drug conjugate being jointly developed and commercialized by AstraZeneca and Daiichi Sankyo.
Study findings coincide with the US government considering recommendations of a second COVID-19 booster dose for adults under 50 years of age.

The FDA has approved daridorexant (Quviviq; Idorsia Pharmaceuticals, US Inc) to treat adults with insomnia.

We have to have this one voice that we've been talking about, this one consistent message.
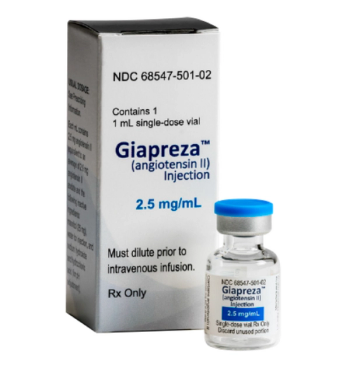
Angiotensin II (Giapreza) is a vasoconstrictor to increase blood pressure in adults with septic or other distributive shock.

Simethicone helps with relief of gas, pressure, and bloating.

Whether it was the ability to vaccinate, whether it was the ability to test, whether it's now the ability to prescribe Paxlovid for patients, those are big progress moves for the profession of pharmacy.

The bill allows pharmacists to dispense and administer drugs, order laboratory tests, and consult patients on HIV pre- and post-exposure drugs.