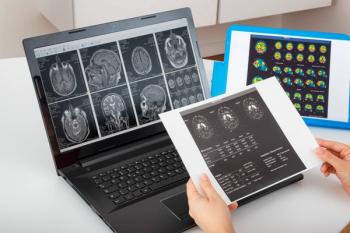
Study results demonstrated that lecanemab provided sustained benefits over 36 months, such as improved biomarker profiles and slowed cognitive decline compared with placebo.

Study results demonstrated that lecanemab provided sustained benefits over 36 months, such as improved biomarker profiles and slowed cognitive decline compared with placebo.
In an analysis of 16 immunocompromised patients with COVID-19, intravenous immunoglobulin was effective and associated with clinical cure.
Afami-cel was approved in conjunction with MAGE-A4 IHC 1F9 pharmDx, a diagnostic tool that can identify patients eligible to receive the treatment for synovial sarcoma.

The CDC says that even if travel is less than 2 weeks away, unvaccinated individuals should still receive a dose of the measles, mumps, and rubella vaccine for protection against the disease.

The diagnostic tool can aid the identification of patients with synovial sarcoma who may be eligible for treatment with newly approved afamitresgene autoleucel.

This is the first and only on-demand, virus-inactivated, human plasma-derived concentration option that is indicated for this approval.
Pharmacists play a key role in the use of live biotherapeutic products, including fecal microbiota, live-jslm for prevention of recurrent Clostridioides difficile (C difficile) infection.
The RUBY trial will continue and analyze the overall population survival after treatment with the drug combination.
Fabry disease is a rare X-linked, lysosomal storage disorder that can cause renal failure or stroke.
The findings provide guidance for clinicians and patients when navigating therapeutic options to establish treatment plans.
Tara Spires-Jones, DPhil, FMedSci, discusses how oligomeric tau clumps inside brain synapses, pointing to indirect evidence that it may be progressing through the brain by jumping between connections.
The co-founder, chief science officer, and chairman at Longeveron discusses the findings from the phase 2a trial CLEARMIND, as well as next steps to developing the therapy Lomecel-B.

Pharmacists' roles in community-based cancer centers are expanding, with a more significant role in managing complex treatment regimens, ensuring comprehensive patient care, navigating drug shortages, and collaborating with multidisciplinary teams to enhance patient outcomes and streamline health care operations.
The drug previously received breakthrough therapy designation for newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia that is in chronic phase.

Community pharmacy leaders should focus on improving quality of work-life for their employees.

The system is an outpatient procedure that resurfaces the mucosal lining, making it easier for the body to maintain healthy metabolism and blood glucose levels.
The designation is based on animal model studies that have demonstrated superior efficacy in comparison to sildenafil.

Tune into this episode of “Public Health Matters” to hear a discussion with Dr. Frank North centered around successful mentorship and advocacy for representation across minority groups in the pharmacy profession.

Tune into this episode of “Public Health Matters” to hear a discussion with Dr. Frank North centered around successful mentorship and advocacy for representation across minority groups in the pharmacy profession.

Understanding and accounting for linguistic diversity is critical in diagnostics and treatment for dementia.

Pharmacies can adapt by embracing digital self-service options to better engage with patients, meet increasing consumer demand, and enhance revenue streams.
The findings indicate that zoster vaccine recombinant reduced dementia risk by 17% in an analysis of over 200,000 patients.

If approved, the drug would be the first in a new class of medications to treat acute pain in over 20 years.

The presence of glutamic acid decarboxylase antibodies in intravenous immunoglobulin can lead to a misdiagnosis of type 1 diabetes mellitus if a false-positive result is garnered.

Joshua Hare describes how Lomecel-B addresses neuroinflammation, unlike other treatments which typically target amyloid disposition.

Patients with MDD may also seek complementary or alternative therapies in the form of dietary supplements which might be perceived by patients to be natural and safer than medicinal agents.

Monitoring OTC sales, dispensing contraceptives, and prescribing birth controls drives a need in policy changes to adapt to increases in pharmacist workload.

The study also found that there were no associations with increased dementia risk when anxiety resolved.
The decision is based on positive results from the PERSEUS trial.

Jennifer Dunphy, DrPH, MBA, MPH and Nelly Awkar-Lazo, MD, discuss the rise in young-onset cancer, the gut-colon cancer link, and the importance of healthy lifestyle choices for cancer prevention.