By decade’s end, approval of additional bispecifics is expected for hematological malignancies, solid tumors, and non-oncology conditions such as inflammatory, autoimmune, neurodegenerative and vascular diseases, and ocular disorders and infections.

By decade’s end, approval of additional bispecifics is expected for hematological malignancies, solid tumors, and non-oncology conditions such as inflammatory, autoimmune, neurodegenerative and vascular diseases, and ocular disorders and infections.
The registry data showed that cardiac magnetic resonance (CMR) was obtained in more clinically complex patients with recurrent pericarditis.
The Auto-Pure 2400 system combines liquid handling and magnetic cell isolation for efficient latent tuberculosis testing.
Pharmacists performed weekly toxicity and tumor lysis syndrome monitoring.

Similar legislation could also address PBM practices in commercial insurance.

Reducing stigma, improving mental health benefits, and addressing workload concerns all need to happen simultaneously.

Evolving breast cancer risk models and prevention methods are necessary to improve patient outcomes, according to Sagar Sardesai, MD.

Current recommended levels of vitamin B12 might not be sufficient to protect against neurological decline.

John Ostrominski, MD shares insights into the safety and efficacy of finerenone, based on data from the FINEARTS-HF trial.
The CDC reports 483 total cases across 20 states as of March 28, 2025.

Sarah Spinler, PharmD, FCCP, FAHA, FASHP, AACC, discussed management of atrial fibrillation in patients with cancer.

M3P will be a financial lifeline for seniors while lowering certain pharmacy costs, but more education and tools are needed to make it successful with pharmacies and their customers.

Host Craig Beavers spoke with several presenters, researchers, and experts about the key data being discussed at the meeting.
Given rituximab’s tendency to cause infections in patients being treated for autoimmune diseases, the addition of intravenous immunoglobulin works to reduce that risk and induce clinical improvements.

The Protecting Pharmacies in Medicaid Act aims to tackle spread pricing, but other legislation could target different issues.

Schizophrenia is a chronic mental illness that significantly affects patients through its symptoms, associated health conditions, and treatment challenges, with long-acting injectable antipsychotics offering potential benefits in improving adherence and reducing relapses.

Anna Sophie Mueller, MD discusses how imaging could advance risk identification in cardiovascular care.

Expert shares insights on leadership resilience, emphasizing self-awareness, team development, and maintaining personal worth beyond professional achievements.

Pharmacists play a key role in COVID-19 management by educating patients on treatment, monitoring drug interactions, promoting vaccination, and addressing hesitancy.
A phase 1/2 clinical trial will provide crucial data on the vaccine's tolerability and pave the way for future development and potential widespread use.
Effective management of immune checkpoint inhibitor-related toxicities requires early intervention, evolving guideline adherence, and multidisciplinary collaboration, with steroids remaining a key treatment.
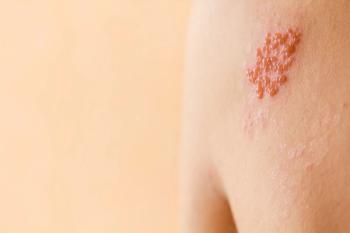
The risk of developing HZ in this population is 10 to 30 times higher than in the general population.

Experts discuss Project Lifeline’s integration of SBIRT in community pharmacies, highlighting its effectiveness in identifying and addressing opioid use disorder while exploring strategies for broader implementation.
The antibody-drug conjugate is currently undergoing evaluation in a phase 1 trial that enrolled patients with pancreatic cancer and squamous non-small cell lung cancer (NSCLC).
Currently, BEAM-302 is undergoing a phase 1/2 clinical trial to evaluate its efficacy in patients with alpha-1 antitrypsin deficiency (AATD) who have lung disease and liver disease.

Marc Humbert, MD, PhD, shared data that were presented at the ACC 2025 Scientific Sessions.

Jawad Butt, MD, offers insight into his analysis of the FINEARTS-HF trial.

John Buse, MD, PhD discusses the expanding role of GLP-1 receptor agonists in treatment of cardiovascular conditions.
Patients in the treatment group had a 21% lower risk of cardiovascular death or first heart failure hospitalization overall, but the between-group difference did not meet the threshold for statistical significance for this primary end point.
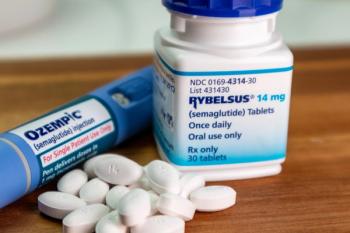
The trial is the first to test the cardiovascular benefits of an oral glucagon-like peptide-1 (GLP-1) agonist.