
Specialty therapies for autoimmune diseases and cancer topped the list of biggest-selling drugs for 2018.

Specialty therapies for autoimmune diseases and cancer topped the list of biggest-selling drugs for 2018.

Study finds patients with negative beliefs about medications may be more likely to experience adverse effects when taking antiretroviral therapy.

This approval is based on results from the Phase III IMpower133 study, which showed that atezolizumab in combination with chemotherapy helped people live significantly longer compared to chemotherapy alone in the intention-to-treat population.
Top news of the day from across the health care industry.

Levoleucovorin injection is being recalled due to the presence of copper salts discovered during a 12-month stability testing.
As a result of the agreement, Teva is licensed to launch a generic version of liraglutide (Victoza, Novo Nordisk) as of December 22, 2023.

Kaiser Family Foundation analysis examines Medicaid outpatient prescription drug utilization and spending before rebates.
Study finds that hepatocytes cells respond to inflammation by activating a protein that creates the environment needed for cancer cells to spread.

Top news of the day from across the health care industry.

What landed this young man in critical condition, in the burn unit?

A recent study showed that flavonoid-rich cocoa improved fatigue by 45% in patients with relapsing and remitting multiple sclerosis.
Recommendations include adopting a heart healthy diet, engaging in exercise, avoiding tobacco, using aspirin sparingly and managing known risk factors.

Having a healthy gut with the right balance of good bacteria may change the entire health of our bodies.

As part of his guilty plea, Calhoun admitted that he defrauded Medicare Part D of $1.6 million through an elaborate scheme in which he created a fictitious pharmacy on paper called “Cal’s Pharmacy” and used it to process hundreds of prescription claims for drugs that were never dispensed.

Over the last 5 years, post-graduate year-1 (PGY1) residency positions have grown by 34% and post-graduate year-2 (PGY2) positions by 64%.

In the March 15, 2019 FluView update, the CDC reported a slight decrease in the percentage of respiratory specimens testing positive for influenza viruses in clinical laboratories, from the previous week.

A recently presented study indicates that while overall HIV mortality is declining, the rate of opioid overdose deaths in HIV patients is increasing.

Back in 1995, Peter Drucker asked, in his book Managing in a Time of Great Change, a very insightful question, "what is your business?" For pharmacy owners today, that question is even more appropriate.

Top news of the day from across the health care industry.

Study provides insight into how prostate cancer transitions to an aggressive, treatment-resistant subtype following anti-androgen therapy.
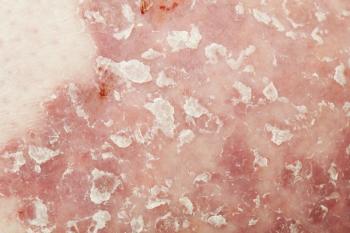
A head-to-head trial evaluated quality of life improvements in patients with moderate-to-severe psoriasis treated with secukinumab (Cosentyx) or ustekinumab (Stelara).

Top news of the week from Specialty Pharmacy Times.
Vaginal inserts containing tenofovir alafenamide fumarate in combination with elvitegravir were shown to be highly effective in preventing simian/HIV infection in a macaque model.


Data show that as many as 4 million adults take a probiotic in the United States, making probiotics some of the most widely used supplements.

Alvotech’s AVT02 is being developed as a biosimilar to the adalimumab (Humira, AbbVie) high-concentration product.

Pharmacy Times takes a look back into the 1920s, when the group of antibiotics was discovered.

Phase 3 trial evaluated ustekinumab as a maintenance treatment in adult patients with moderate-to-severe ulcerative colitis.

Top news of the day from across the health care industry.
Study provides new evidence on the pharmaceutical industry-led medicines access program.