
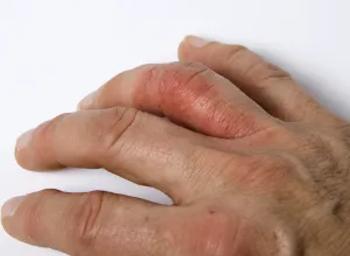
Roflumilast is the first topical agent indicated to treat psoriasis in sensitive intertriginous areas of the skin.
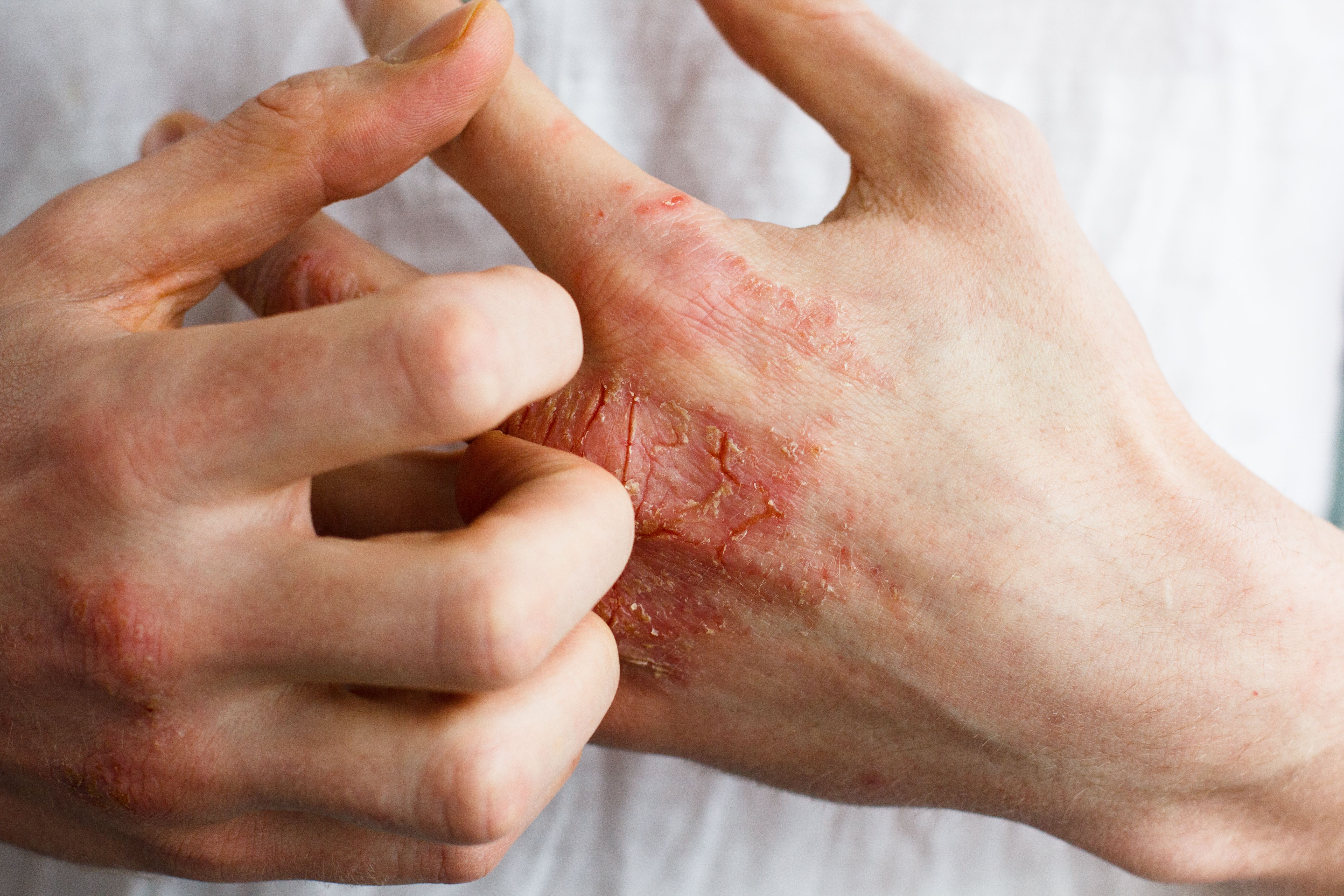

Roflumilast is the first topical agent indicated to treat psoriasis in sensitive intertriginous areas of the skin.
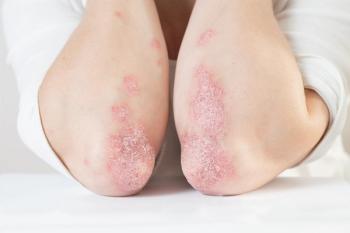
Patients on risankizumab also reported greater satisfaction with treatment for moderate plaque psoriasis.
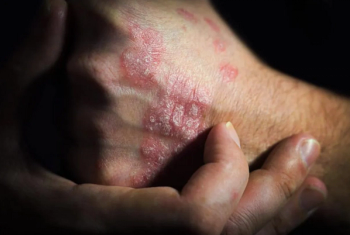
FDA approvals of these biosimilars started in 2016, but only 1 such product has successfully launched.

As more becomes known about Crohn disease, treatment modalities have evolved with biologic monoclonal antibodies becoming a key part of guideline- and evidence-based medicine.

Adalimumab-adbm (Cyltezo Pen; Boehringer Ingelheim) is approved by the FDA as an interchangeable biosimilar to Humira.
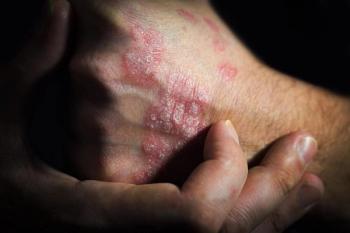
Deuvacracitinib (Sotyktu), which the FDA approved in September 2022, is a first-of-its-class tyrosine kinase 2 inhibitor and a member of the Janus kinase family.
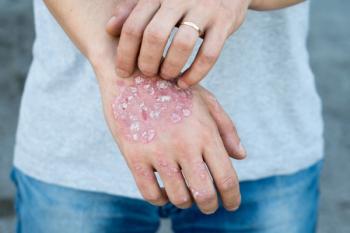
Due to the disease’s chronicity, the variability in treatment response and drug safety profiles, and the availability of multiple therapies, switching or discontinuing treatments is common in psoriasis.
The results of the INSPIRE trial demonstrated that 20.3% of individuals receiving risankizumab (Skyrizi) achieved clinical remission compared to 6.2% of individuals who received the placebo.

Sandoz intends to launch the latest Humira biosimilar in the United States on July 1, 2023.

The drug is indicated for moderate to severe plaque psoriasis.
Several studies have noted a potential link between methotrexate and an increased risk of skin cancer.
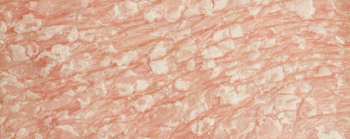
Results from the phase 2 and long-term trials will be presented later this year, following further data collection.
Air pollution from the California Camp Fire are associated with increased rates of clinical; visits, according to new study results.
Deucravacitinib is an oral, selective, allosteric tyrosine kinase 2 inhibitor that inhibits cytokine signaling in psoriasis pathogenesis.

The findings provide an alternative delivery strategy for peptide-based drugs and suggest that such techniques and principles could be applied to a broader range of drugs.
The report highlights the growth of personalized medicines with improved efficacy for rare, previously untreatable diseases.
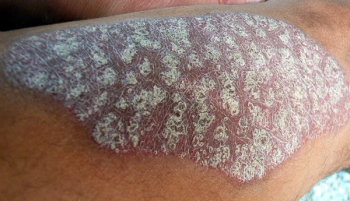
Results show an association between linoleic acid, found in nuts, seeds, and vegetable oils, and increased hypersensitivity in psoriatic lesions.

Clinical trials show that patients with atopic dermatitis experienced improved symptoms with dupilumab starting at 8 weeks, with maximum effect around 12 weeks.
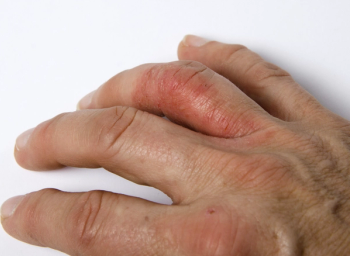
All primary and ranked secondary endpoints of the study were met at week 16, which were sustained or improved through week 24 and were coupled with patient-reported improvements compared with placebo in treating psoriatic arthritis.

Adalimumab-aacf (Idacio) is indicated for chronic conditions such as rheumatoid arthritis, plaque psoriasis, ulcerative colitis, psoriatic arthritis, hidradenitis suppurativa, and juvenile idiopathic arthritis.
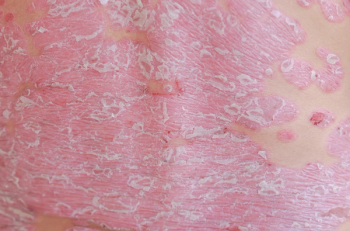
Study finds gluten-free diets may play an important role in the causality of celiac disease on the development of psoriasis.

A modified secukinumab dosing regimen produced outcomes on par with currently recommended intervals in patients with moderate-to-severe psoriasis, but longer follow-ups are needed to confirm the findings.
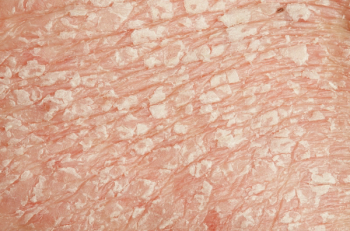
Psoriasis with coexistent lupus erythematosus is uncommon, shows a female preponderance, and is more likely to have joint involvement from both conditions.
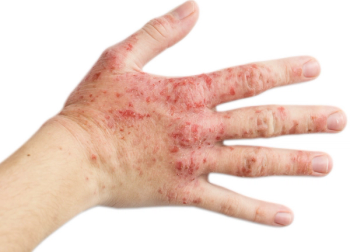
Lipid pathways have been implicated in the pathogenesis of psoriasis and some lipid lowering drugs are believed to have disease-modifying properties.

Treatment with immune checkpoint inhibitors have the potential for immune-related adverse events, such as psoriasis.