IMU-935 is a highly potent and selective inverse agonist of retinoic acid receptor-related orphan nuclear receptor gamma/truncated.


IMU-935 is a highly potent and selective inverse agonist of retinoic acid receptor-related orphan nuclear receptor gamma/truncated.
Research shows that patients with a severe form of psoriasis have a higher risk of death from cardiovascular disease.
The study confirmed that patients with psoriasis are considered to have an increased risk for both chronic kidney disease and ESRD, because inflammation plays a significant role in those conditions as well as heart disease.
Prior evidence regarding the safety and efficacy of the combination of apremilast and phototherapy was lacking, prompting researchers to investigate further.
Janssen’s GUIDE data demonstrate that patients treated with guselkumab less than 2 years after disease onset vs later are more likely to achieve “super-responder” at week 20 through 28.

Deucravacitinib is a selective, allosteric tyrosine kinase 2 (TYK2) inhibitor, which is a member of the Janus kinase (JAK) family.
It is currently indicated for the treatment of adult patients with active psoriatic arthritis and adult patients with oral ulcers associated with Behçet's Disease.
Boehringer Ingelheim’s monoclonal antibody inhibits interleukin-36 (IL-36) signaling and is the first treatment specifically approved for this indication.

Deucravacitinib (Sotyktu) may become the new standard of care oral therapy for moderate-to-severe plaque psoriasis, according to investigators.

Adalimumab-bwwd is a tumor necrosis factor blocker indicated for rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn disease, ulcerative colitis, and plaque psoriasis.

Tapinarof is a first-in-class topical steroid-free aryl hydrocarbon receptor antagonist intended to mitigate the symptoms of plaque psoriasis.

Indications for adalimumab include ankylosing spondylitis, Crohn disease, chronic plaque psoriasis, juvenile idiopathic arthritis, moderate to severe rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.

Tildrakizumab-asmn is a humanized IgG1/k monoclonal anti-IL-23 antibody indicated for the treatment of adults with moderate-to-severe plaque psoriasis.

Long-term extension trial results show a consistent safety profile and durable efficacy for the treatment of adults with the skin condition.

The results of a network analyses show that the elevated potential of contracting the virus is also linked to infliximab and tofacitinib, as well as several combination treatments.

The phase 3, multicenter, randomized, double-blinded, comparative clinical study was evaluating the efficacy and safety of ABP 654 compared with ustekinumab in adult patients with moderate to severe plaque psoriasis.

Deucravacitinib is a possible solution for patients with psoriasis who wish to have the convenience of an oral medication.
Johnson & Johnson’s Janssen Pharmaceutical Companies subsidiary’s post-hoc analysis of Tremfya demonstrates clinically significant improvements.
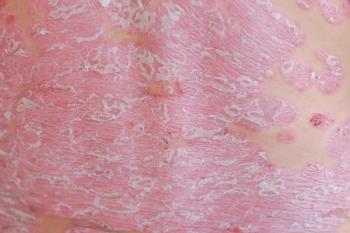
Oral apremilast achieved a clinically meaningful and statistically significant improvement in the primary endpoint of the modified static Physician’s Global Assessment of Genitalia response.

The drug is the first and only oral therapy approved for the treatment of adult patients who are candidates for either phototherapy or systemic therapy.

The study enrolled 214 patients, with 105 randomized to a risankizumab injection every 4 weeks over a 24-week period, while 109 patients received a placebo.
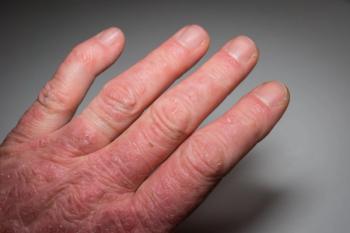
The main factors behind the vaccine hesitancy are the potential adverse effects post-vaccination and effect on their autoimmune conditions, as well as lack of trial data, an analysis shows.