There were approximately 250,000 new cases of prostate cancer identified in the United States in 2021, which is considered approximately 13% of all cancer cases.
Darolutamide Shows Acceptable Long-Term Safety Profile in Nonmetastatic Castration-Resistant Prostate Cancer

There were approximately 250,000 new cases of prostate cancer identified in the United States in 2021, which is considered approximately 13% of all cancer cases.

Men with chronically low testosterone levels are more likely to be hospitalized from COVID-19 than those with normal levels, but testosterone therapy could decrease this risk.

As treatments improve and prostate cancer potentially becomes more of a chronic illness, pharmacists have a chance to shine.

The approval is based on results of the phase 3 study, ARASENS, that demonstrated a statistically significant increase in overall survival, the trial’s primary endpoint.
Preliminary data from the ongoing dose-escalation portion of the clinical trial, show dose-dependent anti-tumor activity when combined with standard-dose Libtayo.

Use of hormone therapy to treat prostate cancer may require more consideration by health care providers as new study shows the therapy may increase the risk of heart disease.
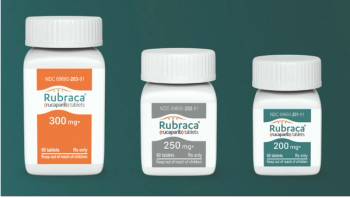
Rucaparib (Rubraca) is a poly (ADP-ribose) polymerase (PARP) inhibitor used for ovarian cancer, fallopian tube cancer, primary peritoneal cancer, and castration-resistant prostate cancer.

Median radiographic progression-free survival was 24.8 months for olaparib plus abiraterone, compared to 16.6 months in the abiraterone arm.

Abemaciclib (Verzenio; Eli Lilly and Company) is an FDA-approved kinase inhibitor being investigated for new indications in individuals with hormone-sensitive prostate cancer and castration-resistant prostate cancer.
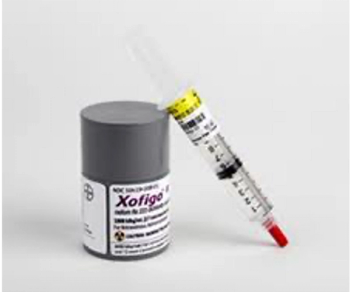
Xofigo is indicated for the treatment of patients with castration-resistant prostate cancer, symptomatic bone metastases, and no known visceral metastatic disease.

In an interview with Pharmacy Times, Amy Pfeifer, PharmD, BCPS, CSP, of AllianceRx Walgreens Prime, discussed the important role that pharmacists can play in the treatment of prostate cancer.

Trials results show darolutamide, docetaxel, and androgen deprivation therapy (ADT) reduced the risk of death by 32.5% versus docetaxel/ADT in patients with metastatic hormone-sensitive prostate cancer.

Blue Earth Therapeutics will begin an open-label, multicenter trial to evaluate a next-generation radiopharmaceutical for metastatic castration-resistant prostate cancer.

Poster at AACR highlights in vitro and in vivosynthetic lethality activity of rucaparib in multiple cancer cell types and PDX tumors that harbor genetic or epigenetic alterations in non-BRCA HRR genes.

Pluvicto is indicated for the treatment of adults with prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer who were previously administered other anticancer therapies.

Lisa Cordes, PharmD, BCACP, BCOP, oncology clinical pharmacy specialist at the National Institutes of Health, discusses treatment options and telehealth for prostate cancer care.

The KEYLYNK-010 trial was evaluating a combination of pembrolizumab (Keytruda) and olaparib (Lynparza) in patients with metastatic castration-resistant prostate cancer who progressed following chemotherapy and either abiraterone acetate (Zytiga) or enzalutamide (Xtandi).

Supplemental new drug application submitted to the FDA for the approval of darolutamide (Nubeqa) plus docetaxel in patients with metastatic hormone-sensitive prostate cancer.
The drug combination reduced the risk of disease progression by 34% versus the standard of care in first-line metastatic castration-resistant prostate cancer, clinical trial results show.
The drug's novel molecular structure may result in fewer toxic adverse effects.
Darolutamide exerts its effects via competitive inhibition of androgen binding.

Xyosted is indicated for conditions associated with a deficiency or absence of endogenous testosterone.

The meta-analysis is the first-of-its-kind phase 3 randomized clinical trial with individual patient data.

The phase 3 ARASENS trial is the only study prospectively designed to evaluate an androgen receptor inhibitor combined with docetaxel and androgen deprivation therapy in this patient population.

Proof-of-contest is inexpensive and quantifies specific antigens from a drop of blood within minutes.